Fifi Zhao, Year 3
Abstract
Despite growing concerns for the adverse health effects of commercial herbicides, these products have soared in popularity following the rise of large-scale agriculture. Likewise, the study of epigenetics has started to be recognized as a new frontier of biology. This study sought to investigate the impacts of glyphosate-based herbicides under an epigenetics lens by extracting protein from yeast exposed to RoundUp, then measuring the presence of H3K27me2 modifications through Western blotting technique. Infrared scanning confirmed a significant presence of the desired H3 protein modification for the control sample, but there was not a detectable amount for the other samples. Further research is needed to determine the exact effects of glyphosate on quantities of H3K27me2 modifications.
Introduction
Epigenetics as a concept has been studied for longer than most would expect – Conrad Waddington, widely credited as the “father of epigenetics” (Noble, 2015), first coined the term in 1942 in his article The Epigenotype (Goldberg et al. 2007). Developmental biologists at first studied epigenetics mainly to investigate the process by which fertilized zygotes mature into organisms (Felsenfeld, 2014). However, recent advances in the field have shown its importance in not only fetal development (Şanlı & Kabaran, 2019), but also cell differentiation (Henning et al. 2018), gene expression (Zhang, 2018) and DNA methylation (Unnikrishnan, 2019). Proposed definitions have reflected these broadening applications, such as “the study of phenomena and mechanisms that cause chromosome-bound, heritable changes to gene expression that are not dependent on changes to DNA sequence” (Deans et al. 2015).
Research has been conducted on a variety of environmental factors that are able to modify the epigenome, such as diet (Herceg, 2007), stress (Bale, 2022), and exposure to chemicals (Tiffon, 2018). Chemicals used in pesticides and herbicides are especially subject to public and professional scrutiny. Interestingly, there is still scientific discourse on whether glyphosate-based herbicides (RoundUp) can potentially be carcinogenic, even after its widespread adoption across the globe (Tarazaon et al. 2017) (Duke and Powles, 2008). Previous studies have looked at human cell datasets to investigate genetic and epigenetic alterations as a result of pesticide exposure (Giambo et al. 2018), as well as how herbicides affect epigenetic processes of meiosis in mice (Gely-Pernot et al. 2015).
Yeast is a widely used model for the human genome thanks to its affordability, accessibility, and relative similarity (Botstein and Fink, 2011) (Liu et al. 2017). Studies have involved yeast in the investigation of epigenetic factors in aging (Pal and Tyler, 2016), the effects of methionine deficiency on epigenetic processes (Sadhu et al. 2013), and the metabolic regulation of epigenetics (Lu and Thompson, 2012), among many more investigations. However, there does not seem to be any comprehensive research done on how the yeast cell epigenome reacts to glyphosate exposure.
Materials and Methods
Yeast Cultivation
Budding yeast, or Saccharomyces cerevisiae, was chosen as the experimental organism. It was the first eukaryotic genome to have its full DNA sequence published and has since become a “model organism” for biologists around the world (Botstein et al. 1997). Fleischmann’s Active Dry Yeast product was used as a starter. Roundup Grass and Weed Control was the selected glyphosate-based herbicide, with a glyphosate concentration of 7g/L. One package of yeast was combined with 50 mL sterile water and incubated at 38°C to activate. 15 mL LB agar was combined with 3 different amounts of herbicide: no herbicide for Group 1, 37 µL for Group 2, and 110 µL for Group 3. Each experimental group had a sample size of 2, and represented a control group, low exposure group, and high exposure group respectively. The volumes of herbicide for each group were based off the low and high ranges of the Manitoba government recommended spray volume for RoundUp (“Agriculture | Province of Manitoba”). 30 µL of the resuspension was added to each agar plate, streaked, and left in a 38°C incubator for 24 hours.
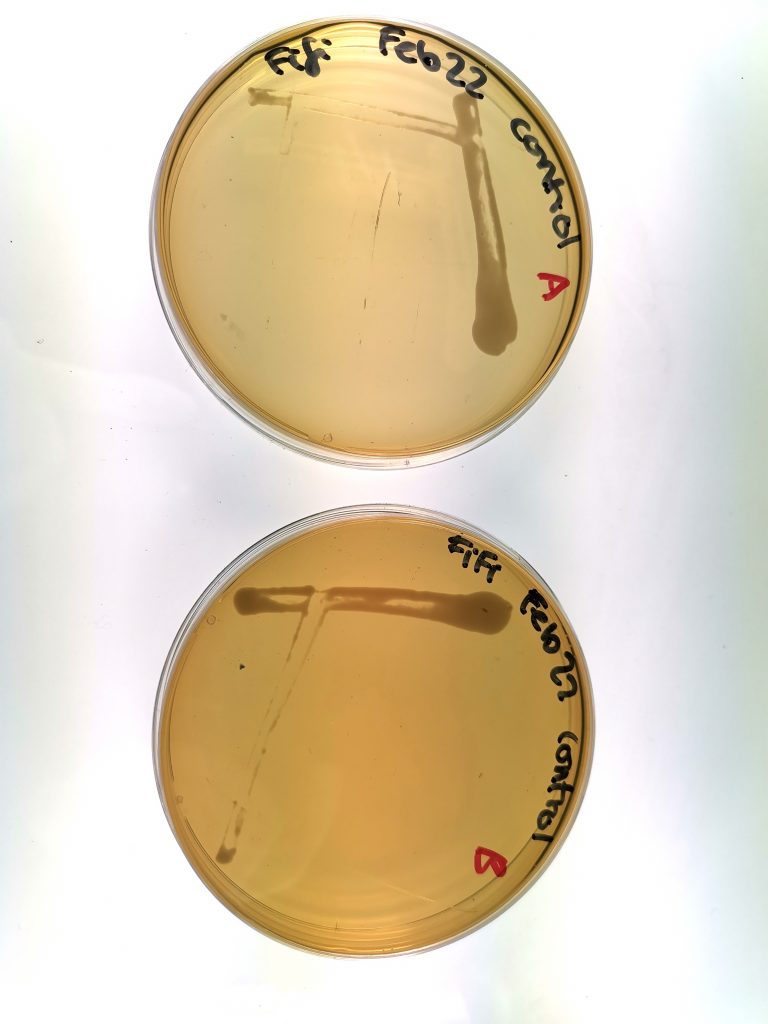
Figure 1. Yeast growth of Group 1 (control group), samples A and B.
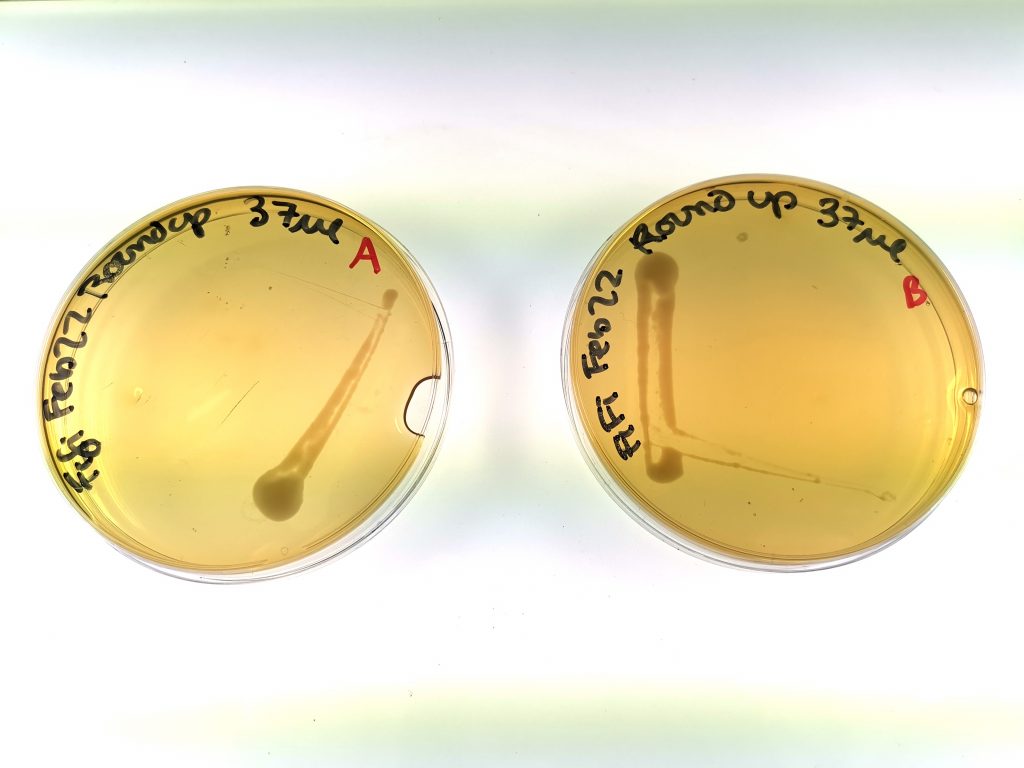
Figure 2. Yeast growth of Group 2 (low exposure group), samples A and B.
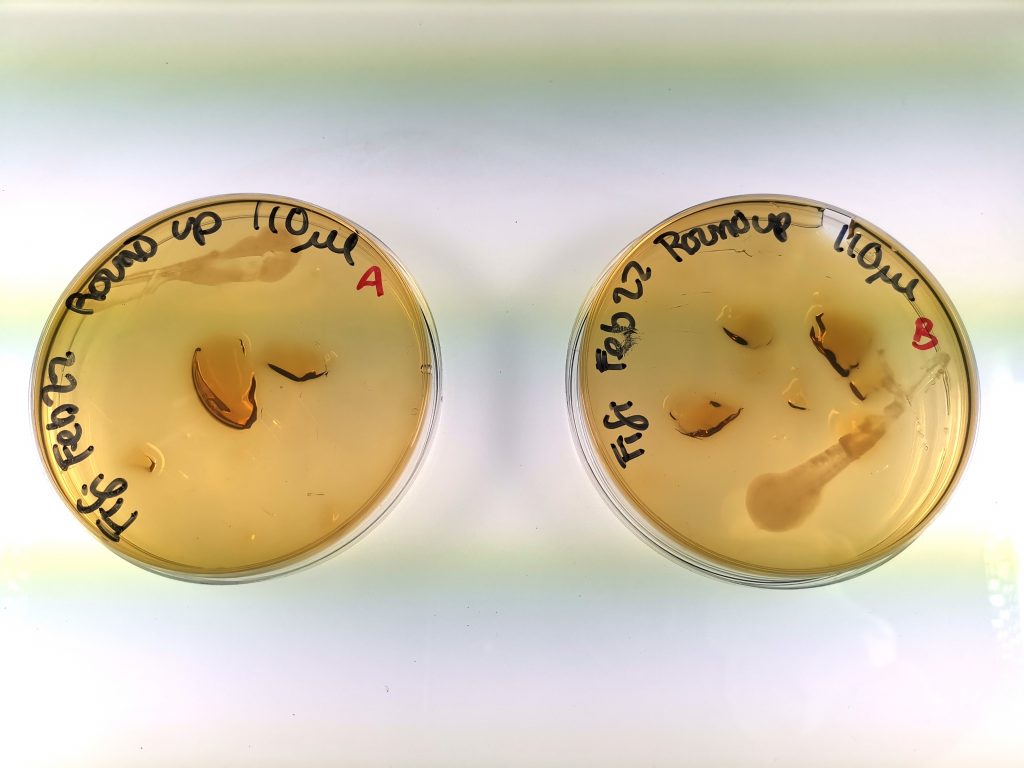
Figure 3. Yeast growth of Group 3 (high exposure group), samples A and B.
Protein extraction
The yeast samples from each plate were added into 1 mL of YPD media, pipetting up and down to resuspend. OD (optical density) of the 6 samples was determined with a Pharmacia Biotech Ultrospec 3000 spectrophotometer, with each cuvette containing 1.9 mL YPD media and 100 µL cell sample. The samples were then normalized. 400 µL of each cell solution was transferred to 1.5 mL Eppendorf tubes and centrifuged at 4400 rpm for 3 minutes. Finally, the supernatant was removed, and the tubes were placed in a -80°C freezer for storage.
Preparation of samples for SDS-PAGE
100 µl dH2O and 100µl 0.2 M NaOH were added to the sample pellet and resuspended. The samples were incubated at room temperature for 15 minutes, then centrifuged at 14,000 rpm for 10 minutes. The supernatants were discarded, 50 µl of 3x SDS sample buffer and β-mercaptoethanol were added in a 9:1 ratio, and the cells were resuspended. The tubes were boiled for 5 minutes in a water bath and centrifuged again at 14,000 rpm for 5 minutes. The supernatants were lastly transferred to a new set of Eppendorf tubes and stored at -20°C, while the pellets were discarded.
SDS-PAGE
The SDS-PAGE was conducted with the Mini Gel Tank by Life Technologies and a 15-well, 4-20% gradient gel by Bio Basic. Tris-Glycine SDS running buffer was poured inside until the gel was submerged. Then, 20 µL of each sample was loaded in, accompanied on both ends by 5 µL of Bio Basic’s 0-250kDa blue-red protein ladder. The SDS-PAGE ran for a total of 60 minutes: first at 30 V until the samples completely entered the gel, then at 200 V for the remainder of the time.
Western Blotting
Western blotting was chosen as the identification method due to the availability of the specific antibodies needed and the visually identifiable results. The Life Technologies Mini Blot Module was used for the transfer of the proteins from the gel to nitrocellulose paper. The gel was placed between a layer of filter paper and sponge pad on both ends and put into the module assembly. Transfer buffer was poured to submerge the module, and the transferring ran at 10 V for 60 minutes.
Figure 4. Samples and gel placed in the module prior to transferring.
The proteins were stained with Ponceau S then submerged in blocking buffer (3% powdered milk in PBST) overnight at 4°C. Following that, the blocking buffer was discarded, and the membrane was rinsed with TTBS and rocked for 5 minutes twice. The antibody added was a rabbit monoclonal antibody for the detection of H3K27me2, and the membrane was incubated overnight and covered on a rocking platform at 4°C. The next day, the primary antibody was discarded, and the membrane was washed with TTBS and rocked for 5 minutes twice again. 1 µL of the antirabbit secondary antibody was added, and the membrane was rocked in the dark for 60 minutes. Finally, it was transferred for visualization to the Odyssey Infrared Imaging System.
Results
The protein ladders separated as intended during the SDS-PAGE, with only mild curvature resulting from the gel. There were also some spillover from the left ladder towards the edge of the gel. The Ponceau S stain revealed that only the first sample showed significant amounts of protein, though the remaining samples are still discernible (see Figure 5). The proteins are concentrated at molecular weights between 70 and 15 kD, with visible bars at 70 kD, 45 kD, 40 kD, 35 kD, and 15 kD.
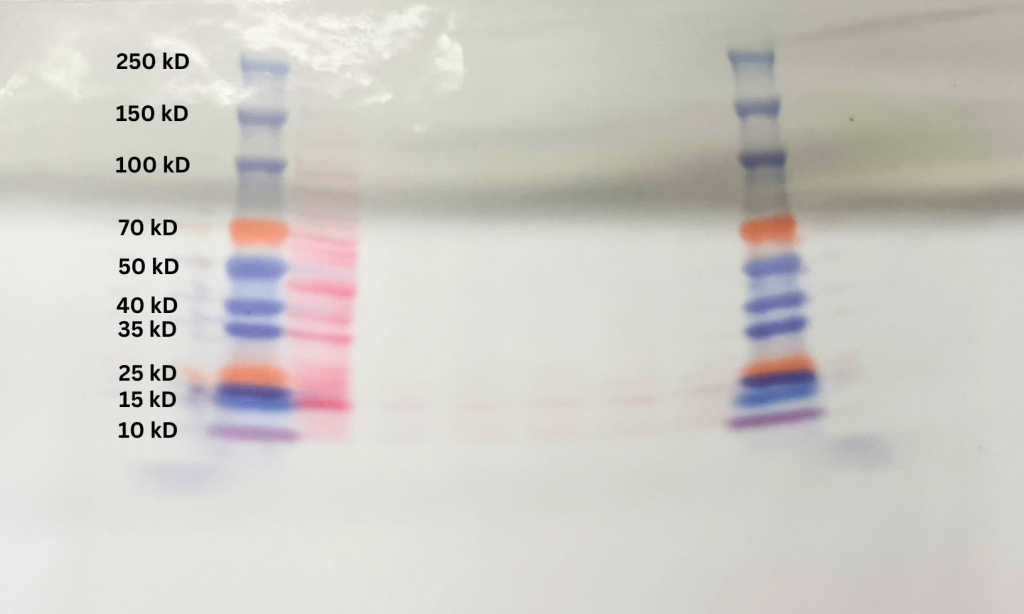
Figure 5. Ponceau S staining (red) of the 6 samples subject to the SDS-PAGE.
Similar to the Ponceau S staining, the imaging did not reveal any significant presence of secondary antibodies except for the first sample (Sample 1A). The visible antibodies seem to be concentrated at the ladder rungs that indicate molecular weights around 20 kD and 10 kD. There is also some spillage around both ladders, and a blotted texture throughout the nitrocellulose membrane.
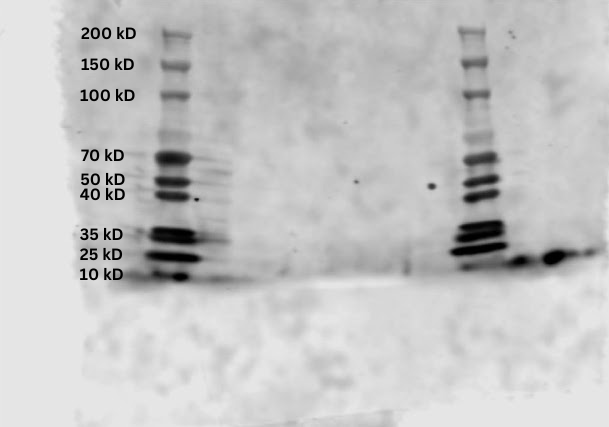
Figure 6. Infrared scan of samples on membrane.
Discussion
The Ponceau S stain suggests that for not enough of the protein was present in the 1B, 2A, 2B, 3A, and 3B samples. Only sample 1A had significant amounts, which could be explained by the normalized procedure conducted during sample preparation. Sample 1A had the lowest amount of cells initially, so the other samples were diluted to normalize all the concentrations. However, OD measurements by spectrophotometers have uncertainties, which may have led to errors in the data. It is possible that the other samples were diluted too much, causing the proteins to be less discernable. Likewise, the low presence of secondary antibodies in the infrared scan suggests there was not enough proteins for the antibodies to attach to in the first place. The blotted texture in the final scan can be attributed to the blocking buffer not fully preventing the primary antibodies from binding indiscriminately to the membrane.
The molecular weights of the proteins indicated by infrared scan do correspond to the average molecular weight of the H3 histone protein, which is 15.3783 kD. This confirms the presence of the histone in Saccharomyces cerevisiae, but unfortunately no further conclusions can be made about the effects of glyphosate-based herbicides on yeast without a comparison to the other experimental groups. That said, the low presence of H3K27me2 does not contradict the results of a similar experiment: when rats were exposed to glyphosate-based herbicides, the H3K27me3 epigenetic modification decreased in presence (Lorenz et al. 2019).
Avenues for improving experimentation include replacing LB agar with liquid medium for yeast cultivation, as well as varying the incubation time, temperature, and glyphosate concentration. Selecting an electrophoresis gel with fewer wells would allow for greater volumes of sample to be analyzed and a higher chance that results are readable. Since the protein ladder rungs were still close to one another towards the lower molecular weights, running the SDS-PAGE for a greater length of time will increase the space between them and improve the precision of readings. Another factor to investigate is the type of herbicide or pesticide with the goal of seeing if their chemical differences can account for different epigenetic modifications. Additionally, since it is possible for yeast to develop a resistance to glyphosate-based herbicides (Ravishankar, 2020), this may be interlinked with any epigenetic response to exposure and is something worth exploring further.
While this study did not achieve its intended purpose of comparing H3K27me2 presence of yeast exposed to different concentrations of glyphosate-based herbicide, it demonstrates the growing importance of epigenetics in research. Herbicides with widespread usage could have lasting impacts on the human genome even if the actual sequencing of DNA is unchanged. It is therefore crucial that future research sheds light on the adverse epigenetic effects of common chemical substances, in order to help regulatory bodies make decisions that protect our world and its future.
References
“Agriculture | Province of Manitoba.” Province of Manitoba, www.gov.mb.ca/agriculture/crops/weeds/print,temperature-and-quackgrass-control-with-roundup.html.
Bale, Tracy L. “Lifetime stress experience: transgenerational epigenetics and germ cell programming.” Dialogues in clinical neuroscience (2022).
Botstein, David, and Gerald R. Fink. “Yeast: an experimental organism for 21st century biology.” Genetics 189.3 (2011): 695-704.
Botstein, David, Steven A. Chervitz, and Michael Cherry. “Yeast as a model organism.” Science 277.5330 (1997): 1259-1260.
Deans, Carrie, and Keith A. Maggert. “What do you mean, “epigenetic”?.” Genetics 199.4 (2015): 887-896.
Duke, Stephen O., and Stephen B. Powles. “Glyphosate: a once‐in‐a‐century herbicide.” Pest Management Science: formerly Pesticide Science 64.4 (2008): 319-325.
Felsenfeld, Gary. “A brief history of epigenetics.” Cold Spring Harbor perspectives in biology 6.1 (2014): a018200.
Gely-Pernot, Aurore, et al. “The epigenetic processes of meiosis in male mice are broadly affected by the widely used herbicide atrazine.” BMC genomics 16 (2015): 1-22.
Giambò, Federica, et al. “Genetic and epigenetic alterations induced by pesticide exposure: integrated analysis of gene expression, microRNA expression, and DNA methylation datasets.” International journal of environmental research and public health 18.16 (2021): 8697.
Goldberg, Aaron D., C. David Allis, and Emily Bernstein. “Epigenetics: a landscape takes shape.” Cell 128.4 (2007): 635-638.
Henning, A. N., Roychoudhuri, R., & Restifo, N. P. (2018). Epigenetic control of CD8+ T cell differentiation. Nature Reviews Immunology, 18(5), 340-356.
Herceg, Zdenko. “Epigenetics and cancer: towards an evaluation of the impact of environmental and dietary factors.” Mutagenesis 22.2 (2007): 91-103.
HHT1 / YBR010W Protein https://www.yeastgenome.org/locus/S000000214/protein (accessed Apr 10, 2023).
Liu, Wei, et al. “From Saccharomyces cerevisiae to human: The important gene co‑expression modules.” Biomedical reports 7.2 (2017): 153-158.
Lorenz, Virginia, et al. “Epigenetic disruption of estrogen receptor alpha is induced by a glyphosate-based herbicide in the preimplantation uterus of rats.” Molecular and cellular endocrinology 480 (2019): 133-141.
Lu, Chao, and Craig B. Thompson. “Metabolic regulation of epigenetics.” Cell metabolism 16.1 (2012): 9-17.
Noble, Denis. “Conrad Waddington and the origin of epigenetics.” The Journal of experimental biology 218.6 (2015): 816-818.
Pal, Sangita, and Jessica K. Tyler. “Epigenetics and aging.” Science advances 2.7 (2016): e1600584.
Ravishankar, Apoorva, Jonathan R. Cumming, and Jennifer EG Gallagher. “Mitochondrial metabolism is central for response and resistance of Saccharomyces cerevisiae to exposure to a glyphosate-based herbicide.” Environmental Pollution 262 (2020): 114359.
Sadhu, M. J., Guan, Q., Li, F., Sales-Lee, J., Iavarone, A. T., Hammond, M. C., Cande, W. Z., & Rine, J. (2013). Nutritional control of epigenetic processes in yeast and human cells. Genetics, 195(3), 831–844. https://doi.org/10.1534/genetics.113.153981
Şanlı, E., & Kabaran, S. (2019). Maternal obesity, maternal overnutrition and fetal programming: effects of epigenetic mechanisms on the development of metabolic disorders. Current Genomics, 20(6), 419-427.
Tarazona, J. V., Tiramani, M., Reich, H., Pfeil, R., Istace, F., & Crivellente, F. (2017). Glyphosate toxicity and carcinogenicity: a review of the scientific basis of the European Union assessment and its differences with IARC. Archives of toxicology, 91(8), 2723-2743.
“The Discovery of the Double Helix, 1951-1953.” National Library of Medicine – Profiles in Science, profiles.nlm.nih.gov/spotlight/sc/feature/doublehelix.
Tiffon, Céline. “The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease.” International Journal of Molecular Sciences, vol. 19, no. 11, Nov. 2018, p. 3425. Crossref, https://doi.org/10.3390/ijms19113425.
Unnikrishnan, Archana, et al. “The role of DNA methylation in epigenetics of aging.” Pharmacology & therapeutics 195 (2019): 172-185.
Zhang, N. (2018). Role of methionine on epigenetic modification of DNA methylation and gene expression in animals. Animal Nutrition, 4(1), 11-16.