Jiayi Wu, Life Science
Abstract
The relationship between pH and the effectiveness of polysaccharides from okra mucilage is analyzed in this study to determine the optimal condition to entrap microplastics. To extract the mucilage from okra pods, the okra is heated using the microwave. Water resistant gauze and a funnel are used to filter the plastic beads in three different samples with three different pH levels (one acidic, one neutral, and one basic). A more acidic polysaccharide solution correlates to an increase in microplastic entrapment. The results can be applied to filtering microplastics in domestic washing machines. A tertiary wastewater treatment is recommended to provide an additional level of treatment to further reduce microplastic pollution. Further research can be conducted to test the effect of detergents or other domestic surfactants on the mucilage to filter microplastics.
Introduction
Plastic debris in the oceans dates to as early as the 1960s (Gill, 2019). Plastics are ubiquitous. In recent years, scientific studies have shown that microplastics form a serious environmental problem (Browne et al. 2011). Microplastics, which are plastics on the order of one micrometer or smaller in size, in water are consumed by marine organisms, and are making their way through the food chain, affecting more and more organisms (Browne et al. 2011).
Microplastics can pose a great risk to the environment, marine organisms, as well as human health. As evidence of this, numerous scientific studies are reporting adverse effects of microplastics on aquatic food webs and on human health. Microplastics come from a variety of sources including but not limited to larger pieces of plastic such as bottles, car tires, and plastic beads in cosmetic products (NOAA, 2023). One main area contributing to the increase in microplastics is the clothing industry. In fact, textiles are estimated to produce about 35 percent of the microplastic pollution in oceans (Mishra et al. 2020). The washing process of synthetic clothes also contributes largely to microplastic pollution due to the friction and turbulence of washing machines that prompt textiles such as clothing and bedding to shed microplastics into microfibers (Okamoto, 2021). According to previous experiments using washing machines from homes to sample wastewater, a piece of garment can yield more than 1900 fibers per wash, validating that a sizable fraction of the microplastic fibres discovered in the marine environment came from sewage as a result of washing clothes (Napper and Thompson 2016). Microplastic pollution of environments and animals is only predicted to rise as the human population rises and people use more synthetic materials.
Leading research conducted and led by Associate Professor Rajani Srinivasan, tested a new way of filtering microplastics from water using okra. Professor Srinivasan and her team found that okra gum, a polysaccharide, had high flocculation efficiency and was therefore considered to be very effective for treatment of sewage wastewater. Available methods of filtering wastewater using non-plant-based flocculant substances prove to be harmful and even carcinogenic through its degradation process (ACS, 2022). Professor Srinivasan and her team looked at okra and plant-based polysaccharides as they hoped to develop plant-based techniques as a way of reducing harmful pollutants in discharge water.
Researchers have investigated water volume on the release of microplastic fibers from laundry, showing that a high-water volume wash, also known as delicate cycles, contribute to a higher release of microfibers compared with a lower water volume wash (Kelly et al. 2019). In addition, research has been done on the effects of extraction pH on the chemical make-up, macromolecular features, and rheological properties of polysaccharide in the case of okra polysaccharide (Bai et al. 2020). The temperature of water when washing clothing has also been suspected to affect microfiber shedding, but there is no sufficient scientific evidence to support this hypothesis. What we do know for certain is that washing with cold water is better for liquid energy use and washing less is better for water consumption (Chiu, 2022).
While results from research have suggested that it is possible to prepare polysaccharides with certain properties and functions, and that the temperature of water may influence microfiber shedding, further research is needed to determine the optimal conditions for filtering microplastics using extracted polysaccharides, of which the pH, temperature of water, and the type of polysaccharide extracted from different sources are significant factors to keep into consideration.
In this experiment, the effect of pH on the entrapment of the microplastic in okra was studied. For the control, the same temperature was used for three samples with different pH. To better represent the temperature of washing clothing, the average temperature of water (22 degrees Celsius) people use when washing clothing was used. This experiment seeks to further contribute to the development of an effective, nontoxic microplastic filtration method to raise awareness and to help reduce wastewater pollution on a personal level.
Materials and Methods
Procedure to prepare okra
Fresh okra with a size of 12-16 cm in length, and approximately 6 cm in perimeter of the center of the okra were stored at room temperature and washed. They are cut into small pieces of about 1-2 cm; both ends discarded. The pieces are placed into a microwavable beaker and 40 ml of tap water is added. The beaker is then placed in the microwave for 20 seconds until the okra is soft. The okra mucilage is stirred using a stir stick. The husk from the mucilage of the okra is separated by using a coffee filter (this process might take an hour as the mucilage is quite viscous). A total of 150 ml is obtained, and the remaining mucilage can be stored in the fridge for use within 2-3 days.
Procedure for filtering the microplastics with mucilage
After the preparation for okra mucilage is complete, it is kept at room temperature. The pH of the mucilage is tested using pH paper (should be around pH of 7). The pH of the water is then adjusted for the experiment (neutral: tap water, basic: 5 ml of bleach, acidic: 5 ml of lemon juice). Three funnels and three pieces of water repellent gauze are used to create a filter for the microplastic beads. The 60 ml of water is poured through along with ⅛ of a cup of microplastic beads through each of the samples and the number of microplastic beads that passed through were counted.
Results
After ⅛ of a teaspoon of microplastic beads were filtered through the gauze and the funnel, the number of beads that flowed through were counted:
Table 1: Data for 4 different pH samples
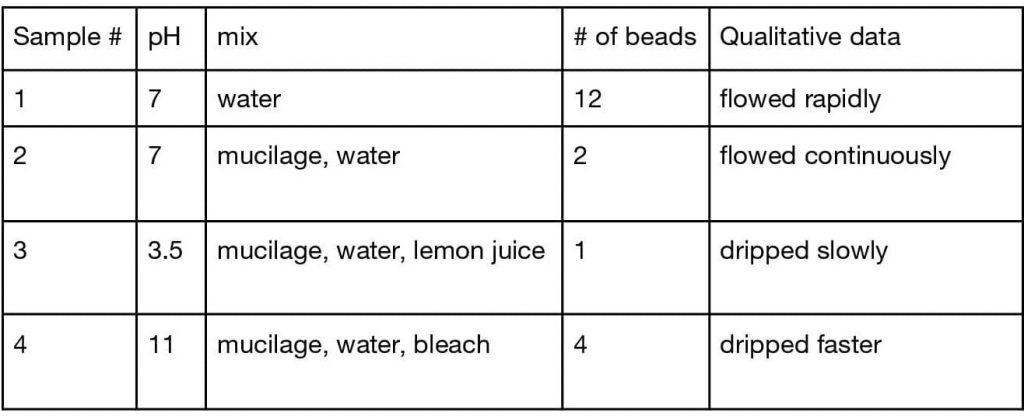
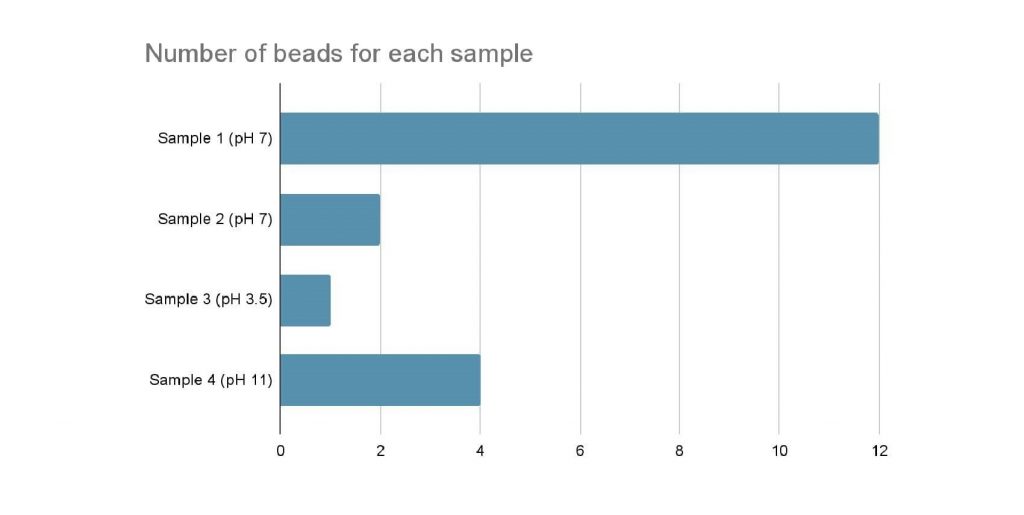
Figure 1: Number of beads for each sample
Discussion
Currently, there are many established methods of filtering wastewater. There is a vast array of laundry filters to address the issues surrounding the shedding of microfibers from washing clothing consisting of both in-drum and external microfiber filters (Okamoto, 2021). There is a filter that you place to the water exit of your washing machine to remove fibres before they reach sewage treatment facilities. There is also a washing-related accessory, like laundry bags made of woven monofilaments with tiny pores or balls that catch microfibers in the wash with your clothes.
Although these filters do help reduce microplastic pollution to a certain extent, they do not solve the problem entirely. Both types require regular cleaning to remove deposits, and to remove plastic from your sewage, you need to dump these fibers into the trash rather than flush them down the sink. Furthermore, plastics will still end up in the landfill, releasing chemicals into the environment and contributing to air pollution. Nonetheless, there is no perfect solution.
Results indicate the more acidic the mucilage, the more entrapment of the microplastic there is. At a molecular level, it appears that the intermolecular hydrogen bonds are at play. Altering the pH of the water can change the viscosity of the mucilage from okra. A lower pH correlates to a higher viscosity and a slower flow rate. Since the viscosity is higher, the mucilage would be more likely to trap microplastics. The stronger the intermolecular hydrogen bonds between the plastic and polysaccharide molecule, the greater the entrapment of plastics. A good description of hydrogen bonding can be found in The Nature Of The Chemical Bond and The Structure Of Molecules and Crystals by Linus Pauling. The result of this experiment corroborates with previous research.
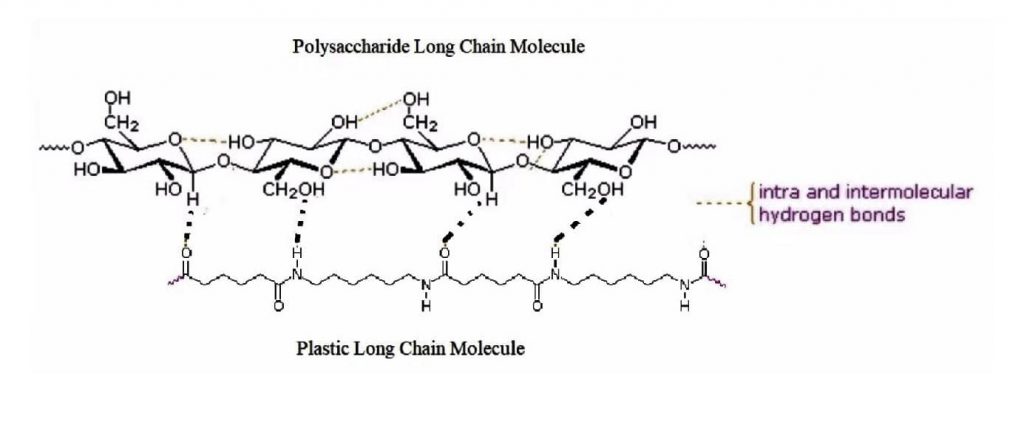
Figure 2: Hydrogen bonds between a polysaccharide and a plastic long chain molecule
To apply this research to washing machines, further research can be conducted to test the effect of detergents or other domestic surfactants on the mucilage to filter microplastics. Currently, as there are no filters for washing machines to prevent debris such as microplastics from flushing out, it may be time to implement a tertiary water treatment to deal with microplastic pollution.
References
American Chemical Society Meeting Newsroom. “Cooking up a way to remove microplastics from wastewater — with okra, aloe.” 22 Mar. 2022. https://www.youtube.com/watch?v=iuOd4aGzHjQ&t=340s
Bai, Lili, et al. “The Influence of Extraction PH on the Chemical Compositions, Macromolecular Characteristics, and Rheological Properties of Polysaccharide: The Case of Okra Polysaccharide.” Food Hydrocolloids, vol. 102, May 2020, p. 105586, https://doi.org/10.1016/j.foodhyd.2019.105586. Accessed 14 Dec. 2022.
Browne, Mark Anthony, et al. “Accumulation of Microplastic on Shorelines Worldwide: Sources and Sinks.” Environmental Science & Technology, vol. 45, no. 21, Nov. 2011, pp. 9175–79, https://doi.org/10.1021/es201811s.
Cox, Kieran D., et al. “Human Consumption of Microplastics.” Environmental Science & Technology, vol. 53, no. 12, June 2019, pp. 7068–74, https://doi.org/10.1021/acs.est.9b01517.
De Falco, Francesca, et al. “Microfiber Release to Water, via Laundering, and to Air, via Everyday Use: A Comparison between Polyester Clothing with Differing Textile Parameters.” Environmental Science & Technology, vol. 54, no. 6, Feb. 2020, pp. 3288–96, https://doi.org/10.1021/acs.est.9b06892.
Gill, Victoria. “Early Ocean Plastic Traced to 1960s.” BBC News, 16 Apr. 2019, www.bbc.com/news/science-environment-47914580.
Kelly, Max R., et al. “Importance of Water-Volume on the Release of Microplastic Fibers from Laundry.” Environmental Science & Technology, vol. 53, no. 20, Aug. 2019, pp. 11735–44, https://doi.org/10.1021/acs.est.9b03022.
Mishra, Sunanda, et al. “Synthetic Microfibers: Source, Transport and Their Remediation.” Journal of Water Process Engineering, vol. 38, Dec. 2020, p. 101612, https://doi.org/10.1016/j.jwpe.2020.101612. Accessed 5 Nov. 2021.
Murphy, Fionn, et al. “Wastewater Treatment Works (WwTW) as a Source of Microplastics in the Aquatic Environment.” Environmental Science & Technology, vol. 50, no. 11, May 2016, pp. 5800–8, https://doi.org/10.1021/acs.est.5b05416.
Napper, Imogen E., and Richard C. Thompson. “Release of Synthetic Microplastic Plastic Fibres from Domestic Washing Machines: Effects of Fabric Type and Washing Conditions.” Marine Pollution Bulletin, vol. 112, no. 1-2, Nov. 2016, pp. 39–45, https://doi.org/10.1016/j.marpolbul.2016.09.025.
National Oceanic and Atmospheric Administration. “What Are Microplastics?” Noaa.gov, 26 Feb. 2021, oceanservice.noaa.gov/facts/microplastics.html.
Okamoto, Katie. “Your Laundry Sheds Harmful Microfibers. Here’s What You Can Do About It.” Wirecutter: Reviews for the Real World, 21 Apr. 2021, www.nytimes.com/wirecutter/blog/reduce-laundry-microfiber-pollution/.
Pauling, Linus. The Nature Of The Chemical Bond and The Structure Of Molecules and Crystals. Oxford University Press, London, 1940.
“Why You Should Almost Always Wash Your Clothes on Cold.” Washington Post, 29 Nov. 2022, www.washingtonpost.com/climate-solutions/2022/11/29/laundry-cold-water-environment/.