In-Woo Park, Life Science
Abstract
Antibiotic resistance has emerged as a critical global crisis, posing a serious threat to modern medicine and society at large. With conventional antibiotics becoming increasingly ineffective, a world with overwhelming antibiotic resistance can have a far greater impact on human life than ever before. The purpose of this study was to discover synergistic antibiotic combinations that inhibits the growth of a gram-negative bacterium: Escherichia coli. In this study, tetracycline, ampicillin, and kanamycin were tested individually and in combination against the E. coli K12 strain at varying concentrations using the broth dilution method. After repeated trials, the combination of tetracycline and ampicillin indicated a significantly synergistic effect, suggesting that the two could improve the fight in combination against antibiotic resistant E. coli.
Introduction
The prevalence of antibiotic resistance is often overlooked by healthcare systems worldwide. Disease-inducing bacteria are increasingly becoming resistant to antibiotics at a faster rate than we can produce them (Bartlett et al. 2013). Without proper intervention, this global crisis can lead to 10 million deaths annually by 2050, with 3 million of those fatalities expected to be caused by one bacterial infection: drug-resistant E. coli (O’Neill 2014). This would also account for more than 40 percent of the cumulative 100 trillion USD lost from world production over the next 35 years (O’Neill 2014).
But even today, antibiotic resistance poses a huge threat and is one of the greatest worldwide challenges to modern medicine and society at large. In 2019, antimicrobial resistance was responsible for at least 1.2 million deaths, with antibiotic resistance alone accounting for 700,000 deaths (Tarín-Pelló et al. 2022). The World Health Organization (WHO) has now classified this crisis as a “serious threat [that] is no longer a prediction for the future.”
To prevent us from further heading into a post-antibiotic era where common infections can be lethal, coordinated efforts must be made to combat this global crisis. This includes further implementing policies such as the Antibiotic Stewardship Pledge (Dellit et al. 2007) or renewing/ investing more into research.
The use of combination antibiotics with synergistic activities have been shown to optimize great results during treatments for bacterial infections (Allen et al. 2002). Synergistic antibiotic combinations are two or more antibiotics combined to form an effect greater than the sum of their predicted individual effects (Kolmer 1948). These combinations allow for lower doses of the constituents followed by considerably enhanced effects (Tallarida 2011). This is achieved by each antibiotic targeting a different aspect of the bacterium, providing a more comprehensive approach to treating the infection (Worthington 2013).
Tetracycline (TET) is a broad-spectrum polyketide antibiotic that binds reversibly to the 30S ribosomal subunit, preventing amino-acyl tRNA from binding to the A-site of the ribosome, thereby inhibiting the bacterium’s protein synthesis (Chopra and Roberts 2001). Comparably, kanamycin (KAN) is an aminoglycoside antibiotic that irreversibly binds to 16S ribosomal RNA of 30s ribosomal subunit, causing interruptions in t-RNA readings that result in inability to synthesize proteins (Franklin and Snow 2005). Ampicillin (AMP) is a β-lactam antibiotic that is used to treat a wide range of infections by interrupting the construction of the bacterium’s cell wall, ultimately leading to the lysis of the bacterium. It achieves this by binding to the bacterium’s primary receptors called membrane-associated penicillin-binding proteins (PBPs) (Tipper 1985).
This study aimed to assess in vitro antibiotic affects of the combinations among tetracycline, kanamycin, and ampicillin against Escherichia coli K12 with the intent of discovering synergistic activity. Considering that ampicillin targets the cell wall for destruction and that both tetracycline and kanamycin target the ribosome to inhibit protein synthesis, it was hypothesized that the combination of ampicillin with either tetracycline or kanamycin would exhibit synergistic antibiotic effects. On the other hand, the combination of tetracycline and kanamycin is hypothesized to have an indifference in antibiotic effects as compared to their individual effects since they both target the ribosome to inhibit protein synthesis.
Materials and Methods
Antibiotics, bacterial strain, and culture media used in antibiotic assays
Tetracycline HCL (TET) (BioShop), ampicillin (AMP) (Merlan Scientific), and kanamycin (KAN) (Merlan Scientific) were used individually as reference antibiotics and in combination against gram-negative Escherichia coli K12. The antibacterial assays were prepared using lysogeny broth (LB) for determining minimum inhibitory concentrations (MIC) and LB agar for determining minimum bactericidal concentrations (MBC).
Determination of minimum inhibitory concentration (MIC)
0.444g of tetracycline HCL powder with a potency of 900µg/mg was diluted with 4mL of LB broth to produce a stock solution of 100mg/mL. The solution was then diluted again with LB broth to produce a working tetracycline solution of 256µg/mL. Similarly, both ampicillin and kanamycin stock solutions were diluted to achieve working solutions of 256µg/mL as well. Combination antibiotics were then prepared by combining equal amounts of volume to form 1:1 ratios.
The wells were filled with 1000µL of individual reference antibiotics and combination antibiotics which were serial diluted with LB broth at concentrations ranging from 0.25µg/mL to 128µg/mL and 0.125:0.125µg/mL to 64:64µg/mL, respectively.
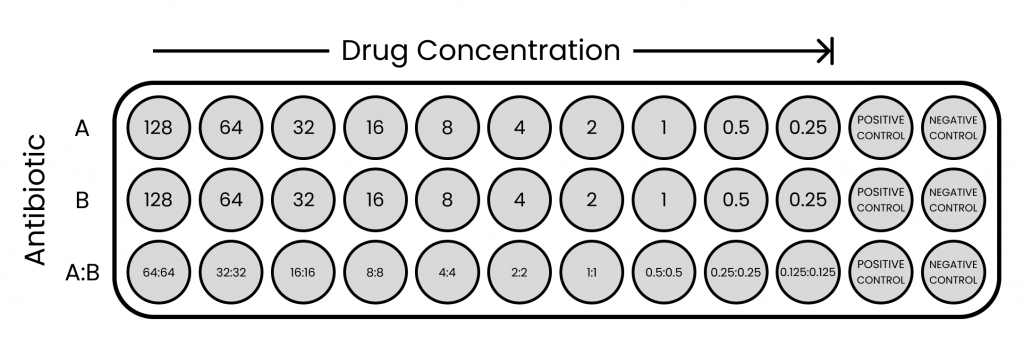
Figure 1. Visual representation of broth dilution assay set up with antibiotic A, B, and A+B combination
E. coli K12 isolates were diluted in LB broth and adjusted to spectrophotometer readings of 0.188 at OD600 to reach a 0.5 McFarland Standard (1.5×108CFU/mL). The prepared inoculum was then diluted again with LB broth to give an inoculum density of 1×106CFU/mL. Then, 1000µL of the inoculum was added to each well plate (except negative control) to give a final inoculum density of 5×105CFU/mL. Blank LB broth was used as the negative control.
The well plates were incubated at 25˚C for 30h then analyzed using Epson® Perfection® V370 Photo to measure assay colour. The MICs of each reference antibiotic and combination antibiotic were defined as the lowest concentrations that showed no growth in the LB broth assay as represented as indifference of colour and turbidity from negative control.
Determination of minimum bactericidal concentration (MBC)
After collecting MIC data, 10µL from both the MIC and the concentration twofold higher, were sampled and inoculated onto LB agar plates which were then incubated at 25˚C for 50h. The MBCs of each reference antibiotic and combination antibiotic were defined as the lowest concentrations that showed no growth on agar.
Statistical analysis for synergism
Antibiotic interactions were assessed algebraically by determining the fractional inhibitory concentration (FIC) index and the fractional bactericidal concentration (FBC) of each combination.
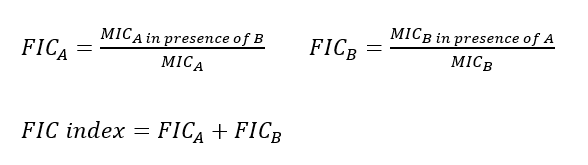
A FIC index of ≤ 0.5 indicates synergism, 0.5 to 4 indicates indifference, and > 4 indicates antagonism where drugs interfere with each other’s mechanism of action, resulting in a weaker effect when used together (Johansen et al. 2000; Hall et al. 1983). The same applies to FBC index.
Results
The results of the MIC and FIC index against E. coli K12 for single reference antibiotics and combination antibiotics were summarized in Table 1., respectively.
Table 1. MIC and FIC index from single reference antibiotics (TET, AMP, KAN) and combination antibiotics against E.coli K12
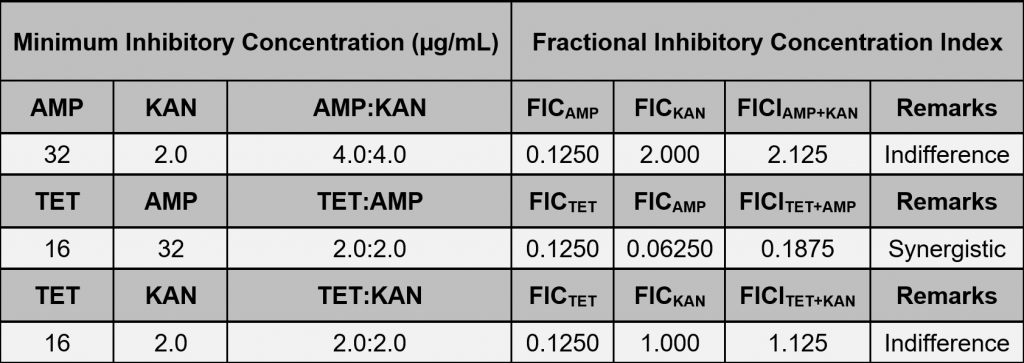
In the broth dilution assay, antibiotic combinations against E. coli K12 showed signs of synergism and indifference from MIC data. The combination of tetracycline and ampicillin were predominantly synergistic with an FIC index of 0.1875. Tetracycline and kanamycin presented an FIC index of 1.125, showing indifference of the combination. Lastly, ampicillin and kanamycin presented an FIC index of 2.125, also indicating indifference.
However, most of the MBC and all FBC index against E. coli K12 for single reference antibiotics and combination antibiotics were inconclusive due to lack of MBC data. All concentrations except 2 single antibiotics showed bacteria growth: tetracycline (32µg/mL) and ampicillin (32µg/mL).
Discussion
The purpose of combination therapy is to enhance antibiotic activity through synergistic interactions of single antibiotics. Utilizing these combinations can treat bacterial infections with lower doses of constituents, potentially saving the healthcare industry trillions of dollars (O’Neill 2014) and revolutionize the way we address antibiotic resistance. Since synergistic combinations require bacteria to develop resistance against two or more different antibiotics, they are profoundly more effective when tackling antibiotic resistance (Gumbo 2015).
In this study, the FIC index for the combination of tetracycline and ampicillin against E. coli K12 was found to be 0.1875, thereby indicating the synergistic effect of the combination, and partially complying with the initial hypothesis. The mechanism involved ampicillin’s inhibition of cell wall peptidoglycan synthesis, increasing the permeability of the bacterium to tetracycline which binds reversibly to the 30S ribosomal subunit and prevents amino-acyl tRNA from binding to the A-site of the ribosome. This comprehensive mechanism synergistically enhances tetracycline’s inhibition of the bacterium’s protein synthesis.
The combination of tetracycline and kanamycin had an FIC index of 1.125, indicating an indifference in antibiotic activity level. This is consistent with part of the initial hypothesis where it was predicted that the antibiotics’ similar mechanism of action may not induce enhanced effects. On the other hand, the combination of ampicillin and kanamycin exhibited an FIC index of 2.125, also indicating indifference and rejecting the initial hypothesis.
It was also observed that the β-lactam antibiotic, ampicillin, was least active when administered against E. coli K12 alone. This can be explained by the bacteria’s production of enzymes called β-lactamases (Jacoby et al. 1988), which also creates the possibility for some degree of resistance to have already existed (Jarlier et al 1988). In addition, it is important to note that the synergistic effects of the antibiotic combinations observed may differ per bacterial strain because of potential differences in the efflux pumps present (Pathania 2019). Some efflux pumps are specific to particular antibiotics or are influenced by the presence of certain substrates/ environmental conditions (Elkins and Mullis 2007). Therefore, this study can benefit from using different strains of E. coli, including antibiotic resistant strains to further investigate the potential for the combination of tetracycline and ampicillin to reduce the advancement of antibiotic resistance of E. coli.
References
Allen, George P., et al. “In Vitro Activities of Quinupristin-Dalfopristin and Cefepime, Alone and in Combination with Various Antimicrobials, against Multidrug-Resistant Staphylococci and Enterococci in an in Vitro Pharmacodynamic Model.” Antimicrobial Agents and Chemotherapy, vol. 46, no. 8, 2002, pp. 2606–2612., https://doi.org/10.1128/aac.46.8.2606-2612.2002.
Bartlett, J. G., et al. “Seven Ways to Preserve the Miracle of Antibiotics.” Clinical Infectious Diseases, vol. 56, no. 10, 2013, pp. 1445–1450., https://doi.org/10.1093/cid/cit070.
Cambrea, Simona. “Antibiotic Susceptibility of Escherichia Coli Strains Isolated in a Pediatric Population from South Eastern Romania.” Journal of Pediatric Infectious Diseases, vol. 09, no. 03, 12 Oct. 2014, pp. 157–162., https://doi.org/10.3233/jpi-140430.
Chevereau, Guillaume, and Tobias Bollenbach. “Systematic Discovery of Drug Interaction Mechanisms.” Molecular Systems Biology, vol. 11, no. 4, 2015, p. 807., https://doi.org/10.15252/msb.20156098.
Chopra, Ian, and Marilyn Roberts. “Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance.” Microbiology and Molecular Biology Reviews, vol. 65, no. 2, June 2001, pp. 232–260., https://doi.org/10.1128/mmbr.65.2.232-260.2001.
De Vries, Henry J, et al. “Efficacy of Ertapenem, Gentamicin, Fosfomycin, and Ceftriaxone for the Treatment of Anogenital Gonorrhoea (NABOGO): A Randomised, Non-Inferiority Trial.” The Lancet Infectious Diseases, vol. 22, no. 5, 19 Jan. 2022, pp. 706–717., https://doi.org/10.1016/s1473-3099(21)00625-3.
Dellit, Timothy H., et al. “Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for Developing an Institutional Program to Enhance Antimicrobial Stewardship.” Clinical Infectious Diseases, vol. 44, no. 2, 2007, pp. 159–177., https://doi.org/10.1086/510393.
Elkins, Christopher A., and Lisa B. Mullis. “Substrate Competition Studies Using Whole-Cell Accumulation Assays with the Major Tripartite Multidrug Efflux Pumps of Escherichia Coli.” Antimicrobial Agents and Chemotherapy, vol. 51, no. 3, 1 Mar. 2007, pp. 923–929., https://doi.org/10.1128/aac.01048-06.
Erol, Erdal, et al. “Synergistic Combinations of Clarithromycin with Doxycycline or Minocycline Reduce the Emergence of Antimicrobial Resistance in Rhodococcus Equi.” Equine Veterinary Journal, vol. 54, no. 4, 4 Sept. 2021, pp. 799–806., https://doi.org/10.1111/evj.13508.
Evans, J., Hannoodee, M., & Wittler, M. (2021, December 15). Amoxicillin Clavulanate. National Center for Biotechnology Information. Retrieved December 13, 2022, from https://www.ncbi.nlm.nih.gov/books
Franklin, T. J., and G. A. Snow. Biochemistry and Molecular Biology of Antimicrobial Drug Action, 31 Oct. 2005, https://doi.org/10.1007/0-387-27566-5.
Gumbo, Tawanda. “General Principles of Antimicrobial Therapy.” Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 12e Eds. Laurence L. Brunton, et al. McGraw Hill, 2015, https://accessmedicine.mhmedical.com/content.aspx?bookid=1613§ionid=102162748.
Hall, M.J., et al. “The Fractional Inhibitory Concentration (FIC) Index as a Measure of Synergy.” Journal of Antimicrobial Chemotherapy, vol. 11, no. 5, 1 May 1983, pp. 427–433., https://doi.org/10.1093/jac/11.5.427.
Jacoby, G. A., et al. “Broad-Spectrum, Transmissible β-Lactamases.” New England Journal of Medicine, vol. 319, no. 11, 15 Sept. 1988, pp. 723–724., https://doi.org/10.1056/nejm198809153191114.
Jarlier, V., et al. “Extended Broad-Spectrum -Lactamases Conferring Transferable Resistance to Newer -Lactam Agents in Enterobacteriaceae: Hospital Prevalence and Susceptibility Patterns.” Clinical Infectious Diseases, vol. 10, no. 4, 1 July 1988, pp. 867–878., https://doi.org/10.1093/clinids/10.4.867.
Johansen, H. K., et al. “Antagonism between Penicillin and Erythromycin against Streptococcus Pneumoniae in Vitro and in Vivo.” Journal of Antimicrobial Chemotherapy, vol. 46, no. 6, 1 Dec. 2000, pp. 973–980., https://doi.org/10.1093/jac/46.6.973.
Kizirgil, Ahmet, et al. “In Vitro Activity of Three Different Antimicrobial Agents against ESBL Producing Escherichia Coli and Klebsiella Pneumoniae Blood Isolates.” Microbiological Research, vol. 160, no. 2, 25 Apr. 2005, pp. 135–140., https://doi.org/10.1016/j.micres.2004.10.001.
Kolmer, J. A. The synergistic or additive activity of chemotherapeutic compounds. Am J Med Sci. 1948 Feb;215(2):136-48. PMID: 18898012.
Mawabo, Isabelle K., et al. “Tetracycline Improved the Efficiency of Other Antimicrobials against Gram-Negative Multidrug-Resistant Bacteria.” Journal of Infection and Public Health, vol. 8, no. 3, May 2015, pp. 226–233., https://doi.org/10.1016/j.jiph.2014.09.001.
Olajuyigbe, OO, and AJ Afolayan. “In Vitro Synergy and Time-Kill Assessment of Interaction between Kanamycin and Metronidazole against Resistant Bacteria.” Tropical Journal of Pharmaceutical Research, vol. 14, no. 5, 2 Oct. 2015, pp. 837–843., https://doi.org/10.4314/tjpr.v14i5.14.
O’Neill, Jim. Government of the United Kingdom, 2016, pp. 10–63, Tackling Drug-Resistant Infections Globally: Final Report and Recommendations.
Pathania, Ranjana, et al. “Efflux Pump Inhibitors for Bacterial Pathogens: From Bench to Bedside.” Indian Journal of Medical Research, vol. 149, no. 2, Feb. 2019, pp. 129–145., https://doi.org/10.4103/ijmr.ijmr_2079_17.
Singh, Nina, and Pamela J Yeh. “Suppressive Drug Combinations and Their Potential to Combat Antibiotic Resistance.” The Journal of Antibiotics, vol. 70, no. 11, 6 Nov. 2017, pp. 1033–1042., https://doi.org/10.1038/ja.2017.102.
Tallarida, R. J. “Quantitative Methods for Assessing Drug Synergism.” Genes & Cancer, vol. 2, no. 11, 2011, pp. 1003–1008., https://doi.org/10.1177/1947601912440575.
Tarín-Pelló, Antonio, et al. “Antibiotic Resistant Bacteria: Current Situation and Treatment Options to Accelerate the Development of a New Antimicrobial Arsenal.” Expert Review of Anti-Infective Therapy, vol. 20, no. 8, 2022, pp. 1095–1108., https://doi.org/10.1080/14787210.2022.2078308.
Tipper, Donald J. “Mode of Action of β-Lactam Antibiotics.” Pharmacology & Therapeutics, vol. 27, no. 1, 1985, pp. 1–35., https://doi.org/10.1016/0163-7258(85)90062-2.
Wallace, R. J. (1984, December 26). Amoxicillin-Clavulanic Acid in the Treatment of Lower Respiratory Tract Infections Caused by -Lactamase-Positive Haemophilus influenzae and Branhamella catarrhalis. American Society for Microbiology, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC180185/pdf/aac00184-0046.pdf
Wang, Lei, et al. “Synergistic Activity of Fosfomycin, Ciprofloxacin, and Gentamicin against Escherichia Coli and Pseudomonas Aeruginosa Biofilms.” Frontiers in Microbiology, vol. 10, 6 Nov. 2019, https://doi.org/10.3389/fmicb.2019.02522.
“Who’s First Global Report on Antibiotic Resistance Reveals Serious, Worldwide Threat to Public Health.” World Health Organization, World Health Organization, 30 Apr. 2014, https://www.who.int/southeastasia/news/detail/30-04-2014-who-s-first-global-report-on-antibiotic-resistance-reveals-serious-worldwide-threat-to-public-health.
World Health Organization, 2014, Antimicrobial Resistance: Global Report on Surveillance.
Worthington, Roberta J., and Christian Melander. “Combination Approaches to Combat Multidrug-Resistant Bacteria.” Trends in Biotechnology, vol. 31, no. 3, 18 Jan. 2013, pp. 177–184., https://doi.org/10.1016/j.tibtech.2012.12.006.
Xu, Xuejie, et al. “Synergistic Combination of Two Antimicrobial Agents Closing Each Other’s Mutant Selection Windows to Prevent Antimicrobial Resistance.” Scientific Reports, vol. 8, no. 1, 8 May 2018, https://doi.org/10.1038/s41598-018-25714-z.
Zhou, Alice, et al. “Synergistic Interactions of Vancomycin with Different Antibiotics against Escherichia Coli: Trimethoprim and Nitrofurantoin Display Strong Synergies with Vancomycin against Wild-Type E. Coli.” Antimicrobial Agents and Chemotherapy, vol. 59, no. 1, 23 Dec. 2014, pp. 276–281., https://doi.org/10.1128/aac.03502-14.
Zhou, Yuling, and Yan Peng. “Synergistic Effect of Clinically Used Antibiotics and Peptide Antibiotics against Gram-Positive and Gram-Negative Bacteria.” Experimental and Therapeutic Medicine, vol. 6, no. 4, 23 July 2013, pp. 1000–1004., https://doi.org/10.3892/etm.2013.1231.