Jessie Liu, Year 2 Research
Abstract
When the coronavirus first broke out, people began to create their own hand sanitizers through tutorials as store-bought hand sanitizers ran out. Most tutorials propose the addition of external ingredients, yet, there is little research of whether they prove to be effective. Consequently, the shortage of these products can risk others who are medically dependent on them. This experiment serves to test the effects of these additional ingredients and their effect on bacteria inhibition. Results suggest little to no significant difference between each ingredient, however, glycerin overall had the widest zone of inhibition, with aloe vera and orange lemon zest ranging the smallest zone of inhibition. This suggests that some ingredients can actually lower the effectiveness of the hand sanitizer.
Introduction
The Coronavirus immediately induced a panic, and people all over the world began purchasing hand sanitizers to reduce the spread of disease, some even purchasing in bulk. Consequently, hand sanitizers are running out in stores and as an alternative, people are starting to make their own DIY hand sanitizers, searching up on the web for tutorials. Many websites propose the idea of adding other ingredients such as aloe vera gel, essential oils, vegetable glycerin, vitamin E oil, or orange lemon zest. While some of these, such as essential oils and aloe vera, do exhibit microbial and anti-viral properties, there is yet much research on whether these ingredients actually add value to hand sanitizers (Nazarro 2013; Athiban 2012).
Some people are medically dependent on these extra ingredients, such as aloe vera gel, as it contains antifungal properties that can help soothe their injuries; if others are purchasing them to make hand sanitizers, this can result in a shortage of this gel. Recent mutations of the coronavirus further extend the pandemic and induce fear of health. Moreso, the risk of future possible global diseases is possible. Thus, as the usage of hand sanitizers is long-term, if the extra ingredients proves to add no value to hand sanitizers itself, then we risk preventing the people who truly need the gel to others who purchase it merely to follow the online DIY tutorials.
Isopropyl alcohol is to be most effective against pathogens due to its amphiphilic properties, similar to the phospholipid membrane that protects almost all cells (Berg 2002). The alcohol molecules are essentially able to bond and penetrate through the membrane, then denature the cell from the inside. As the proteins break down, their structure is lost, and the cell cannot function properly. Thus, the bacteria lose their phospholipid bilayer barrier, dehydrate, and die.
While many of the extra ingredients do have antimicrobial properties, such as aloe vera, there is another possibility that adding these extra ingredients may actually make the hand sanitizer less effective as compared to the original (Athiban 2012). Moreso, there is other similar research, such as Trivedi’s article, that tests the efficacy of aloe vera gel as a disinfectant (2019). However, it does not test its effect within hand sanitizers, but aloe vera gel in itself. Chakraborty’s research, on the other hand, does include all the extra ingredients in hand sanitizer, but he focuses on the economic aspect, rather than the effectiveness to eliminate bacteria (2021). There is yet a study to be found that tests the effectiveness of such a vast number of ingredients to its effectiveness in hand sanitizer. Thus, this experiment attempts to provide a more diverse investigation upon such additional ingredients and their effectiveness against bacteria.
Materials and Methods
This experiment hopes to conduct research on the effects of additional ingredients to alcohol hand sanitizers on the potency of bacteria elimination. Specifically, it will test five different ingredients, with a control group of 80% diluted alcohol: aloe vera gel, tea tree essential oil, vitamin E oil, vegetable glycerin, orange lemon zest. Each product will be created using 80% alcohol hand sanitizer, soaked in a disc and then placed in an agar plate distributed evenly with the Pseudomonas fluorescens bacteria before incubation.
The five hand sanitizers containing a different additional ingredient were created. Each product (tea tree oil, vitamin E oil, orange lemon zest) was created using 50mL of the 80% alcohol. However, the aloe vera gel and vegetable glycerin were mixed with 99% alcohol to create an overall 80:20 ratio of alcohol and the extra ingredient, respectively. 10 drops of tea tree oil and vitamin E oil were used to create their sanitizers, and the orange lemon zest was taken from shredding the peels of the orange and lemon zest using a grater, dropping 2 tablespoons of each fruit and mixing them into the alcohol base. After creating each product, they were placed inside a plastic jar, labeled according to their ingredients, including a control group of the 80% alcohol.
Table 1: Brief description of how each hand sanitizer product was created.
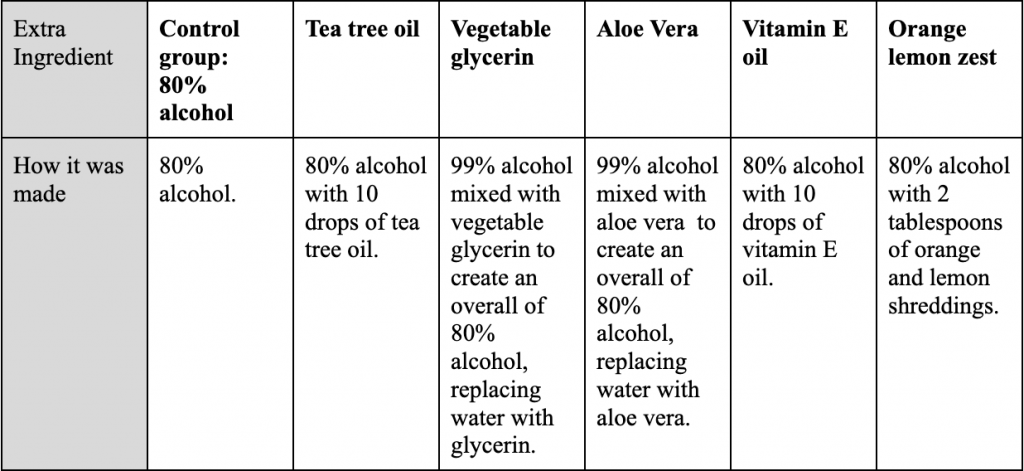

Figure 1: Hydrometer in 80% alcohol.
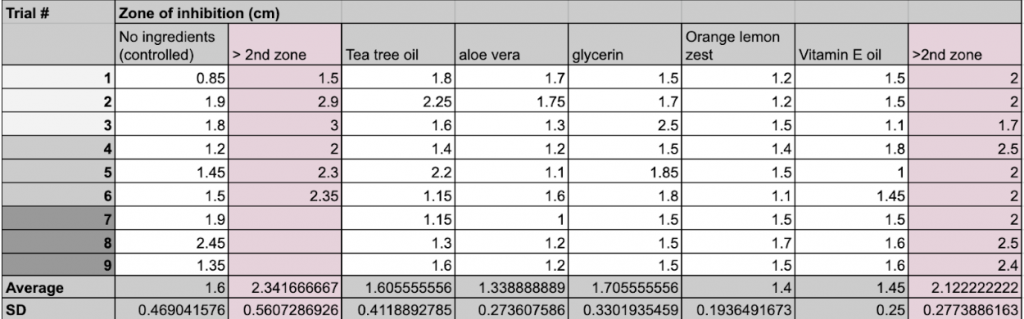
A total of 18 agar plates were prepared accordingly for the experiment. The bacteria Pseudomonas fluorescens was used to plate each agar plate using the Disk Diffusion Method. Each petri dish was labeled with a black marker of the bacteria used, type of ingredient, and divided into three even sections.
Each hand sanitizer had 9 trials, using a total of 3 agar plates. First, a sterilized tweezer was used to grab a 6mm disk and soaked into the desired solution for about 5 seconds. Then, the disk was removed from the solution and the excess liquid was shaken off. After, the petri dish labeled the proper ingredient was opened, and the disk was placed in the center of one of the three sections. This process was repeated until all sections were filled with one disk, and the same task followed suit to the other plates. When finished, the plates are placed inside the incubator, stacked on top of each other, and incubated for a week.
After one week, the petri dishes were taken out of the incubator, and placed side by side on a table. A clear 15cm ruler and a black marker was then used to measure the diameter of the zone of inhibition for each trial, including the disk itself. Each quantitative and qualitative observation was recorded on a table.
Results
The quantitative data was inputted in a table, then the average and standard deviation was calculated as shown below.
Table 2: Raw data of each individual trial per ingredient with average and standard deviation.
As shown in Table 2, a second zone of inhibition was prominent in the controlled group as well as the Vitamin E oil trials. This was noted, however, these 2nd zones were not taken into account in the graph.
The qualitative observations shown in Figure 2 presents each plate as transparent, with a noticeable zone of inhibition shown by a circle around the disk. The control group and the vitamin E oil group had two clear zones of inhibition, however, some trials within the control were not as clear, thus not measured and inputted. Every trial of glycerin had a noticeable yellow pigment.
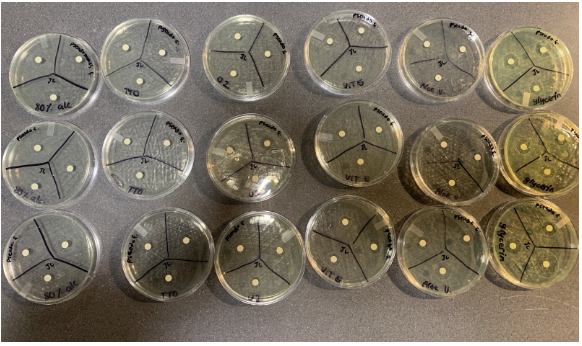
Figure 2: All 18 trials of the ingredients.
Figure 3: Graph showing average and error bars of the effects of additional ingredients in alcohol-based hand sanitizer on the zone of inhibition of Pseudomonas fluorescens.
As suggested in Figure 3, glycerin had the largest average zone of inhibition out of all the other groups. Compared to the controlled group’s diameter of inhibition, tea tree oil exhibited little difference, and the additional ingredients of aloe vera, orange lemon zest, and vitamin E oil had an overall smaller zone of inhibition. In fact, aloe vera had the lowest average of all of the groups. As suggested through the error bars, there was a large amount of overlap between the trial results.
Discussion
In summary, the results suggest that, compared to the control group, glycerin had the largest average zone of inhibition, tea tree oil had little to no difference, and vitamin E oil, orange lemon zest, and aloe vera had the lowest average.
While each ingredient has evidently demonstrated to be antibacterial, the results suggest that certain ingredients are more effective in bacteria inhibition than others. Glycerin, in particular, suggests to be the most effective due to its moisturizing properties. Since alcohol has a boiling point of 78.37 °C, it evaporates very quickly, creating an immediate coagulation of the surface of the cell wall (National Center). When this happens, it creates an external injury that will form a protective wall around the cell, shielding the organism and preventing the alcohol from penetrating the cell walls (Golin 2020). Glycerin, on the other hand, is highly hygroscopic, meaning it absorbs moisture from the air, and has a very high boiling point of 290 °C. Glycerin’s organic compound contains many OH molecules, meaning it can create a hydrogen bond with the alcohol compound (National Center). Hydrogen bonding is one of the strongest intermolecular bonds, thus, it mixes with alcohol, and prevents the immediate coagulation, and lets the alcohol penetrate inside the bacteria cell, killing the bacteria (Bylikin 2014). While some of the other ingredients also have moisturizing properties, they are not as effective, as their structure limits the number of hydrogen bonds, as well as their boiling point is not as high as glycerin. In addition, glycerin left a noticeable yellow stain to the nutrient gel. This is because glycerin is susceptible to oxidation, and at a certain decomposition rate, it turns yellow, thus this qualitative observation is not a reason for its high average of bacteria inhibition (National Center).
Yet, overall, the difference of the results between each ingredient are not drastic, as well as the high overlap of the error bars, meaning that results are most likely not significant. Part of this reason can be due to the 80% alcohol percentage, as this means there is distilled water that would help prevent the alcohol from drying too quickly. Moreover, the results suggest that some ingredients in fact lower the effectiveness of the hand sanitizer, as both aloe vera and the orange lemon zest demonstrated a lower average zone of inhibition as compared to the control group. With this information, someone who is using 99% alcohol hand sanitizer could consider adding glycerin to get a more effective and long lasting disinfectant.
One limitation to this experiment was the lack of trials conducted at different times. While nine trials per ingredient do seem abundant, these are all done at the same time, meaning that if there were perhaps any issues with the incubator during the week, all the petri dishes would be affected, thus inaccurate results. To ensure this is not an issue, trials should be done more than once at different times. A possible human error was the length in which each disk was soaked in the solutions. As the time immersed was not counted, perhaps the disks were unevenly soaked, resulting in some disks having more solutions than the others. This can result in higher variance in the zone of inhibition, as some disks had more time to dry than the others. As a solution, the duration of the disks being soaked should be time so it can be confirmed that it is not a contributing factor to the error bars. Another human error can be the amount of times the bacteria has been plated. As for the Disk Diffusion Method to distribute my bacteria evenly, the number of times the bacteria was being plated was not counted, but approximately determined by plating in a few turns until a full circle. As the degrees turned are not consistent, more bacteria could be placed in certain petri dishes than others, resulting in uneven bacteria growth and skewed results. To fix this problem, next time the degrees and number of turns should be calculated to ensure each bacteria is evenly plated per dish.
Overall, much more research needs to be conducted to have a meaningful and confident conclusion in the effectiveness of adding extra ingredients, especially what kind of extra ingredients. For further investigation, another trial on a different bacteria should be conducted, as hands contain many different types of bacteria, and perhaps these results are not universal to all bacteria. Additionally, different percentages of alcohol should be used, such as 90% alcohol to determine if results differ, as the main reason for this experiment’s results is due to dryness. As there were no other similar works that could be found—either they were focused on a different topic or the ingredients were not as diverse—this experiment provides a new scope that other scientists could look into.
References
Allott, A., & Mindorff, D. (2014). IB Biology Course Book: 2014 Edition: Oxford IB Diploma Program (2014 ed.). Oxford University Press.
Athiban, Prakash P et al. “Evaluation of antimicrobial efficacy of Aloe vera and its effectiveness in decontaminating gutta percha cones.” Journal of conservative dentistry : JCD vol. 15,3 (2012): 246-8. doi:10.4103/0972-0707.97949
Berg, J. M. (2002). By Jeremy M. Berg – Biochemistry, Fifth Edition: International Version (hardcover) (Fifth Edition) (2002–03-02) [Hardcover] (5th ed.). W. H. Freeman.
Bylikin, Sergey, et al. Oxford IB Diploma Program Chemistry: Course Companion. 2014 ed., Oxford University Press, 2014.
Golin, Andrew P et al. “Hand sanitizers: A review of ingredients, mechanisms of action, modes of delivery, and efficacy against coronaviruses.” American journal of infection control vol. 48,9 (2020): 1062-1067. doi:10.1016/j.ajic.2020.06.182
Huffer, S., Clark, M. E., Ning, J. C., Blanch, H. W., & Clark, D. S. (2011). Role of alcohols in growth, lipid composition, and membrane fluidity of yeasts, bacteria, and archaea. Applied and environmental microbiology, 77(18), 6400–6408. https://doi.org/10.1128/AEM.00694-11
National Center for Biotechnology Information. “PubChem Compound Summary for CID 702, Ethanol” PubChem, https://pubchem.ncbi.nlm.nih.gov/compound/Ethanol. Accessed 26 March, 2022.
Nazzaro, Filomena et al. “Effect of essential oils on pathogenic bacteria.” Pharmaceuticals (Basel, Switzerland) vol. 6,12 1451-74. 25 Nov. 2013, doi:10.3390/ph6121451