Michelle Liu, Year 2 Research
Abstract
Scientific dental biofilm research is vital to improving dental care. However, it is challenging to extract dental biofilms from patients’ teeth. Pseudomonas fluorescens was grown on a 24-well plate in liquid colonies, and acetic acid was applied to the colonies in varying amounts. The amount of biofilm in each well is quantified by using the safranin staining method. As a result, the qualitative data shows that 50 𝜇l acetic acid produced the most amount of Pseudomonas fluorescens biofilm, and 200 𝜇l acetic acid produced the least amount of Pseudomonas fluorescens biofilm.
Introduction
Our teeth play one of the most essential roles in our life. They are what we need to break down food items for digestion. Dental biofilm is a community of living microbes that attaches to the subgingival or supragingival surfaces of the teeth and grows into microcolonies. When consuming carbonated food and drinks, bacteria feed on the sugars and produce acids that cause problems like tooth decay. The majority of the time, a balance is maintained in the oral ecosystem, but when the bacteria becomes overly abundant, problems start to occur. It is important to study the best way to prevent and treat them. Dental caries and periodontal diseases are closely related to the formations of biofilm that result from microbial colonization of the tooth surface (Seneviratne, Zhang, and Samaranayake, 2011). Current studies show the growing demand for research regarding tooth protection and dental biofilms. One study pointed out that Biofilm infections contribute to up to 80% of human microbial infections and are associated with common human disorders, such as diabetes mellitus and poor dental hygiene (Bjarnsholt, T et al. 2018). The use of actual teeth in research raises problems such as costs, accessibility, consent, and safety. A study has shown that the storage of extracted teeth as they require a precise sterile process that must be neutral for the enamel and dentin microstructure because even a minor change can affect the adhesive bonding (Nawrocka and Lukomska-Szymanska, 2019).
Materials and Methods
Growing the Bacteria
A sterile pipette tip was used to gently streak the surface of the agar. Two plates were left in the incubator overnight.
A colony from the two plates was chosen and grown overnight in the LB broth. The Pseudomonas Fluorescens (16-24 hours) were grown at 30 ℃, and the 15ml culture tubes must be placed under 37 ℃ to prevent pathogens from growing and the container must be sterile.
Diluting the Bacteria
The liquid bacteria culture was diluted with a 1:100 dilution in a 24 well plate with 1mL volume in each well with the below samples
Table 1: Experimental Groups
Capturing Results with Staining
Safety considerations of using safranin include wearing goggles, tying hair back, wearing a lab coat and closed-toe shoes. First, supernatants were removed from each well by a pipette. The wells were then air-dried for 30 mins and safranin 0.1 % was added to each well to a point where it was above where the cultures grew. Wait for 15 mins and collect the stains by a pipette. The stains were then placed into a waste container labeled safranin stain. Each well was then washed 3 times and removed by shaking out the water over a waste container. Lastly, the plate was placed upside down on a paper towel to dry for 30 mins. The plates were then imaged using a camera.
Results

Figure 1: Pseudomonas fluorescens
Table 2: Safranin stains in each well
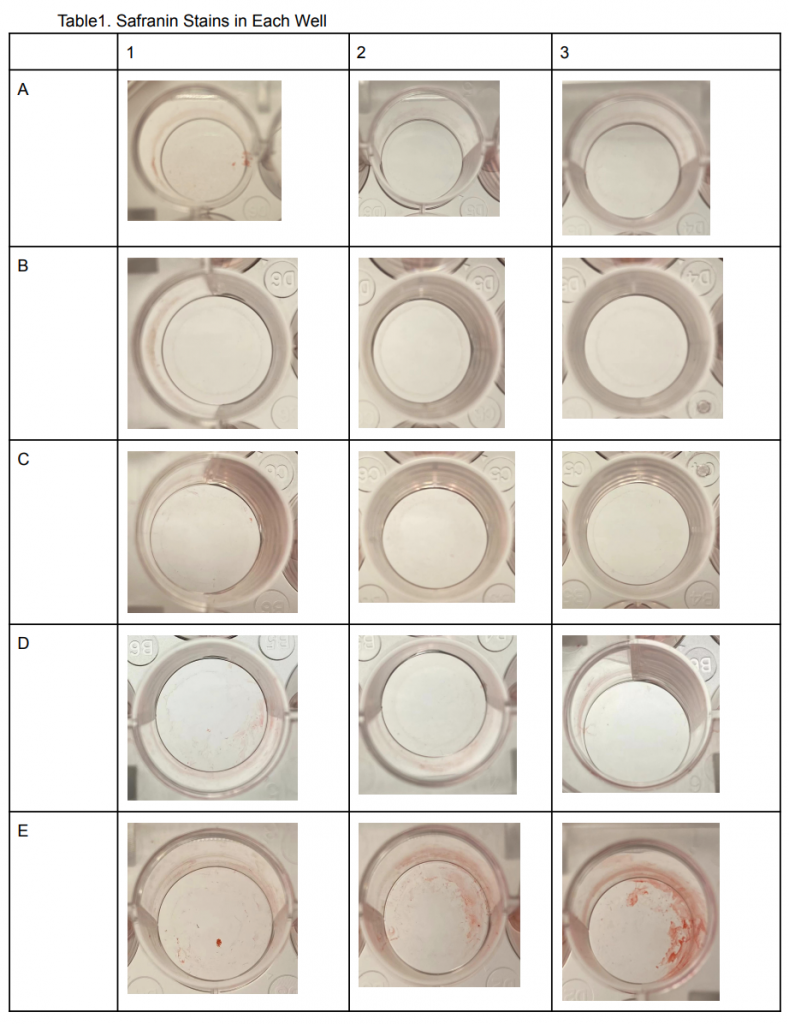
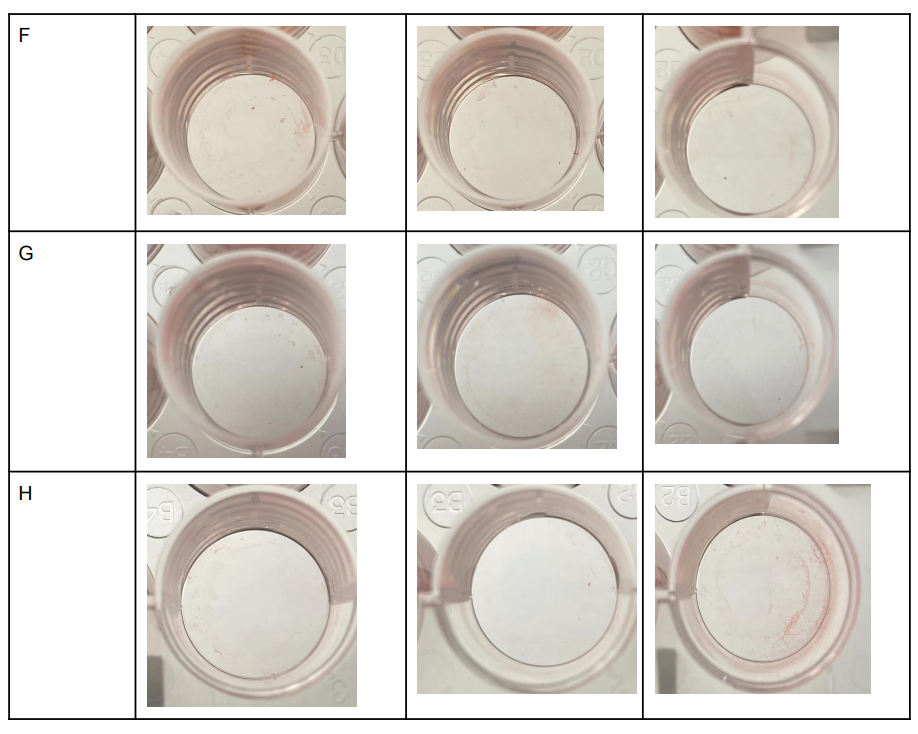
As Shown in Table 2. A1, A2, and A3 seemed to have little to no safranin stains inside the wells. This was predictable because the first roll was A1-3 wells were the negative control with no bacteria grown inside the wells. This was also seen in B1, B2, and B3 wells since it was also a negative control that had E.Coli grown inside the wells. In contrast, the red color seemed to be practically gone in C and D1-3 wells even though Pseudomonas fluorescens were grown in these wells with no acetic acid. In wells E1-3, the safranin colors seem to be most intense despite having 50 𝜇l of Acetic acid treatment, a similar result can be seen in wells F1-3. Wells G1-3 and H1-3 on the other hand showed significantly less safranin in the wells almost similar to A1-3.
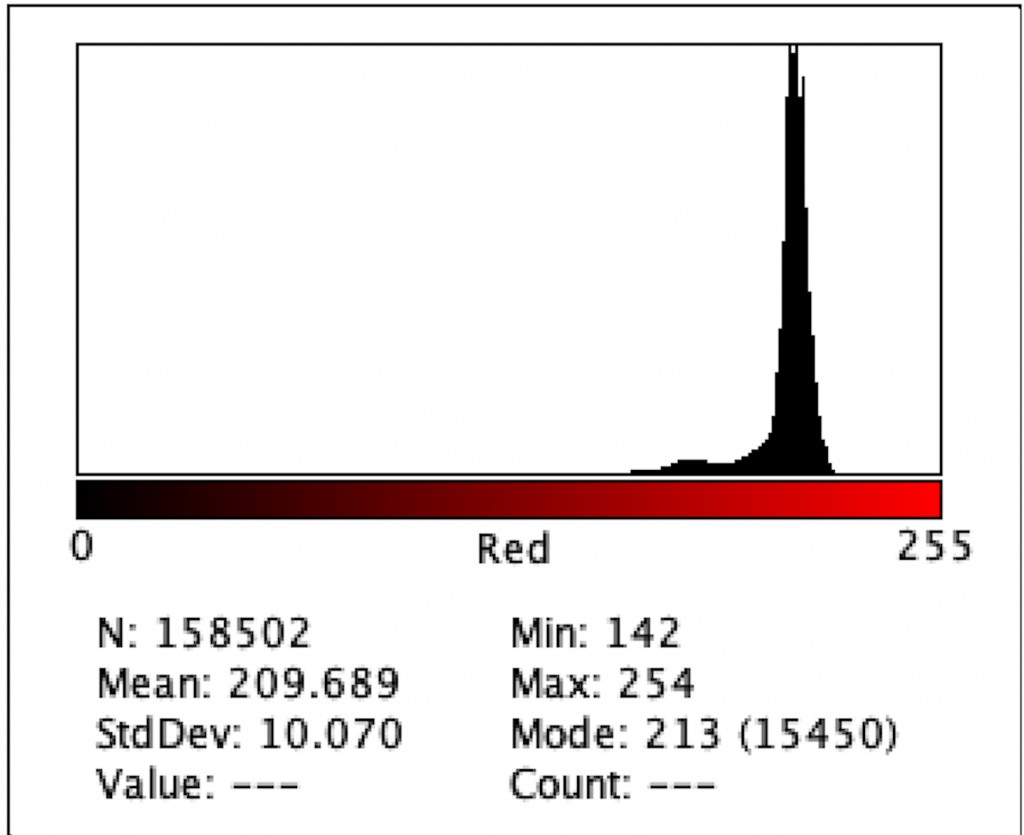
Figure 2: Intensity of Red Color for A1
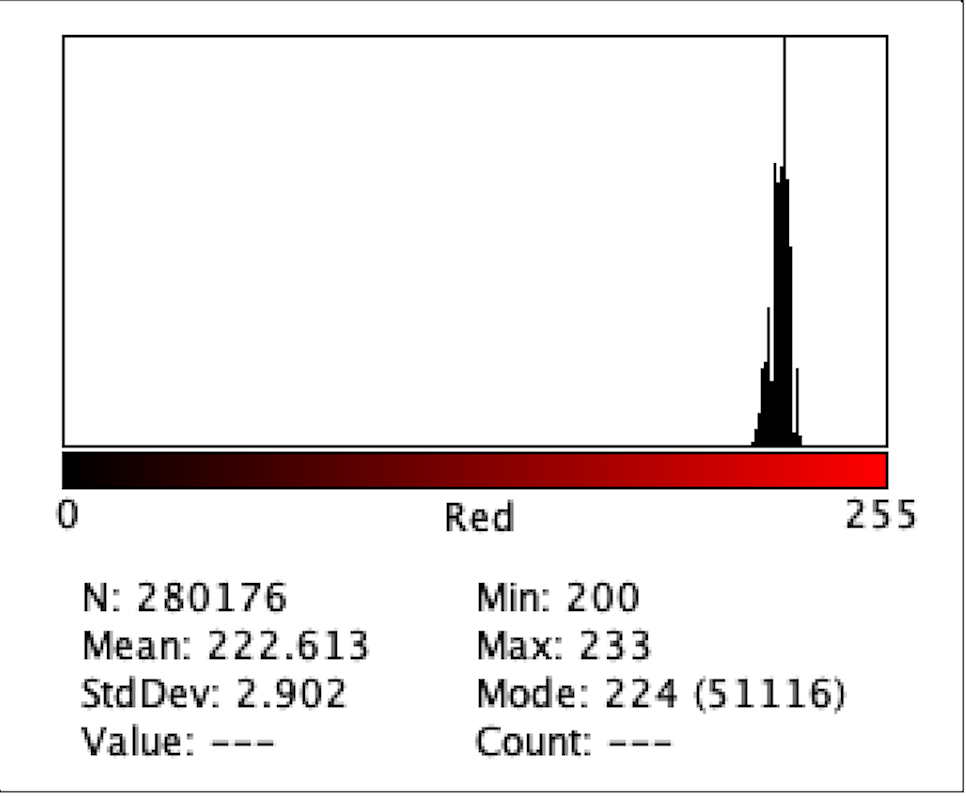
Figure 3: Intensity of Red Color for B3
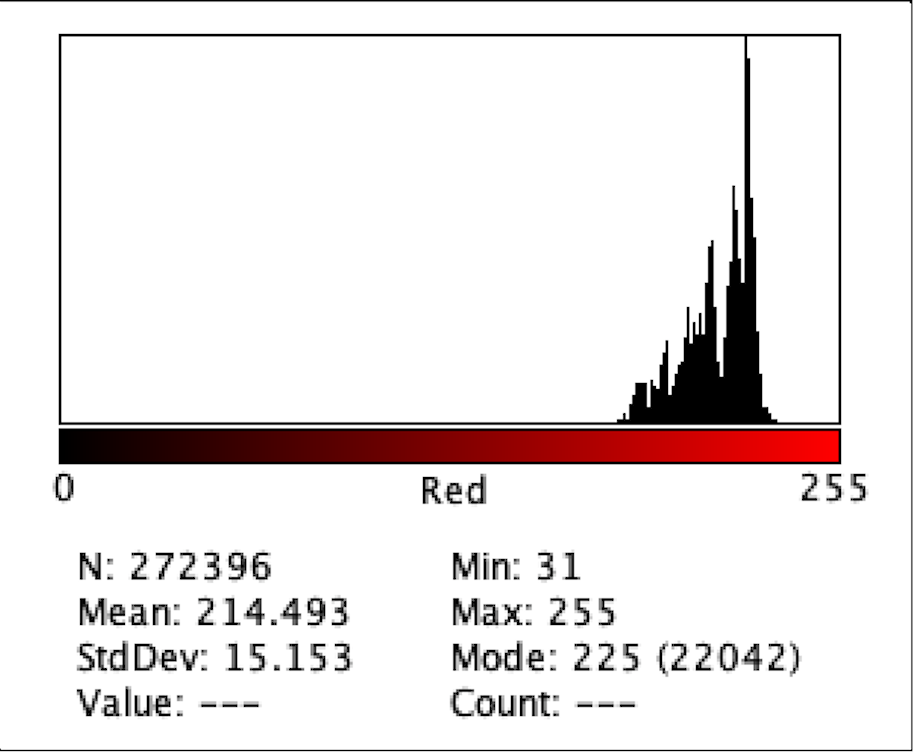
Figure 4: Intensity of Red Color for C1
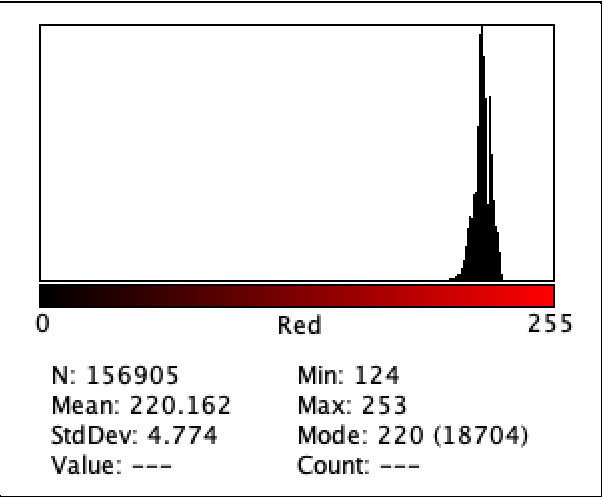
Figure 5: Intensity of Red Color for D1
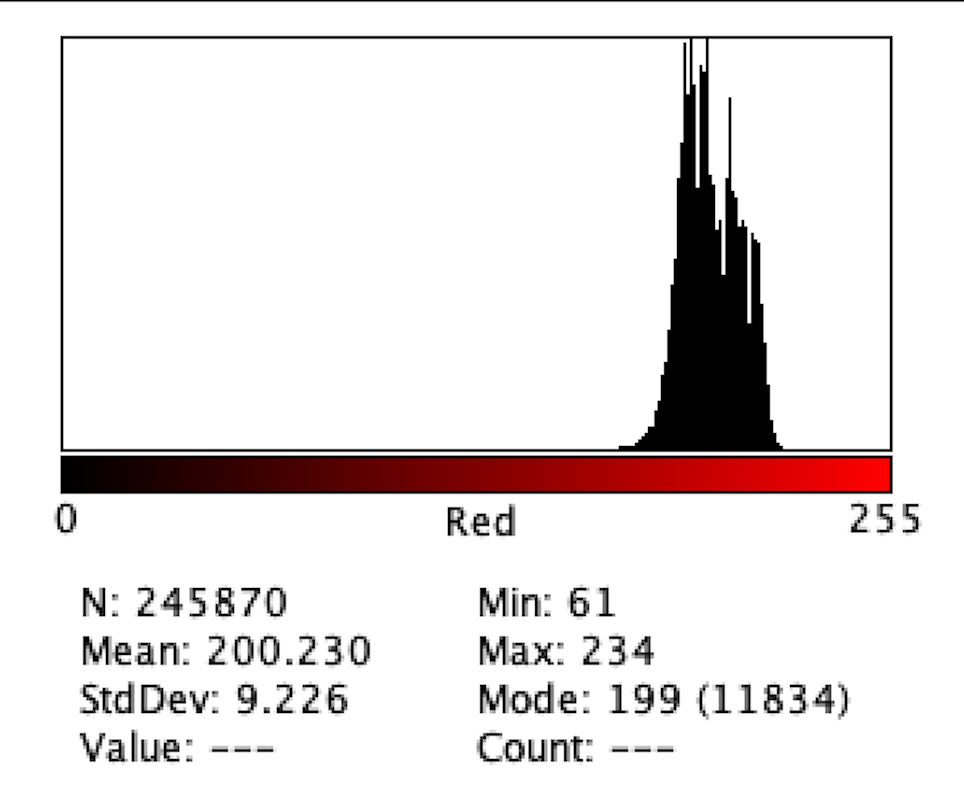
Figure 6: Intensity of Red Color for E2
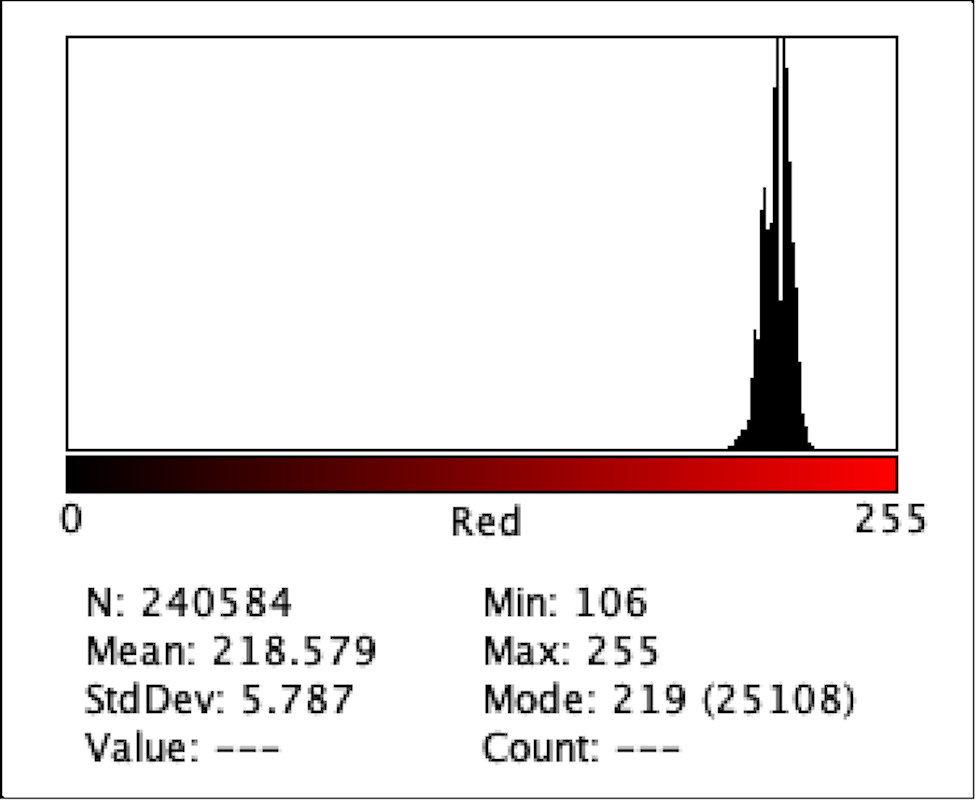
Figure 7: Intensity of Red Color for F1
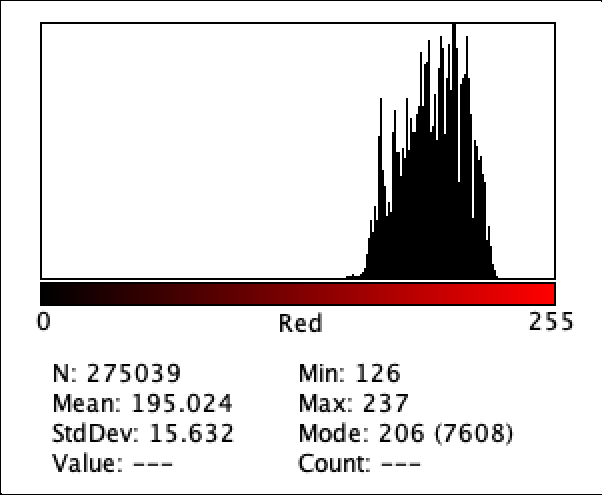
Figure 8: Intensity of Red Color for G1
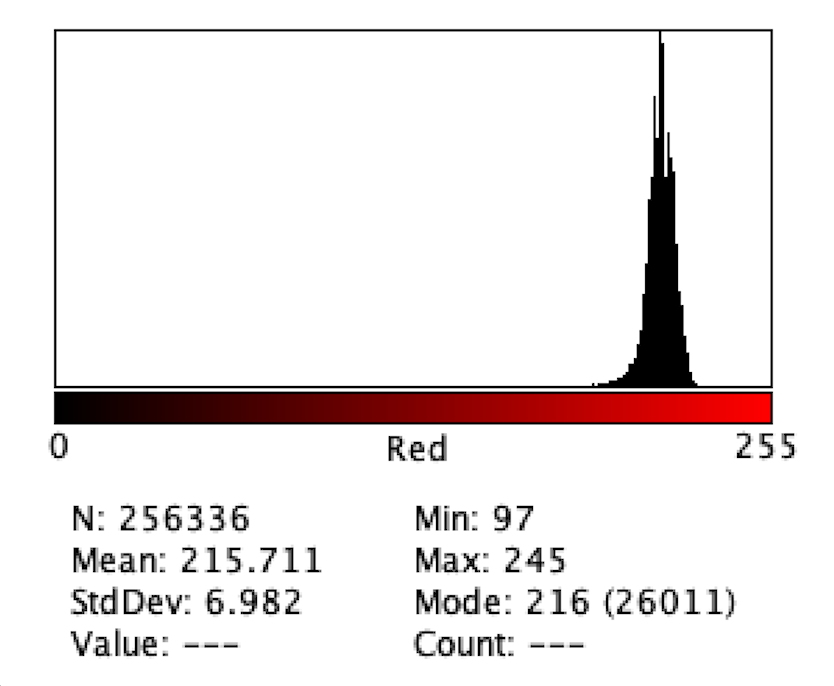
Figure 9: Intensity of Red Color for H3
Figure 2 to Figure 9 show the analysis of the intensity of red color in each well using the app Imagej. According to the analysis, the mean was the highest in the well B3 (222.613), and in descending order: D1 (220.162), F1 (218.579), H3 (215.711), C1 (214.493), E2 (200.230), G1(195.024), where G1 has the lowest intensity of the color red.
Discussion
In terms of qualitative data, in wells E1-3, the safranin colors seem to be most intense despite having 50 𝜇l of Acetic acid treatment. However, Imagej shows that the B1-3 wells have the most intense red color. It is possible that because the pictures for each well have varying red tones, ImageJ picks up the background color along with the red safranin color in the photos. This could potentially interfere with the mean value that is displayed for each color intensity histogram. In the future, a better imaging technique could be implemented to better visualize the biofilms in each well. Furthermore, because of the lack of time, only one treatment, Acetic acid, was tested for the biofilm. The project originally planned to test other treatments such as garlic extract and N-acetylcysteine. With more time in the future, these other liquids could be applied.
Furthermore, biofilms grown in wells are in very different conditions compared to those in the oral cavity. Future experiments could include further mimicking of the oral cavity in order to ensure that the biofilms tested are grown under similar conditions. In the oral cavity, the dental pellicle needs to be deposited on tooth surfaces for oral biofilm to develop. It is mostly composed of salivary proteins. In order to mimic this coat, some authors recommend using artificial saliva. (Lopez-Nguyen and Badet, 2016) Since the use of actual human teeth may cause problems such as cost, ethical and legal issues. hydroxyapatite could be used as a substitute to mimic dental tissues. (Lopez-Nguyen and Badet, 2016)
Bacterial species include Fusobacterium nucleatum, Treponema spp, Tannerella forsythensis, P. gingivalis, Aggregatibacter actinomycetemcomitans, et al attach to mature biofilms after they form corn cob forms, bristle brush forms or other forms. (Huang et al. 2011) The bacteria strain used for this experiment is Pseudomonas fluorescens which is not typically found in the oral cavity, thus future experiments could use the above strains of bacteria mentioned.
References
Amankwah, Stephen et al. “Bacterial Biofilm Destruction: A Focused Review On The Recent Use of Phage-Based Strategies With Other Antibiofilm Agents.” Nanotechnology, science and applications vol. 14 161-177. 14 Sep. 2021, doi:10.2147/NSA.S325594
Bjarnsholt T, Buhlin K, Dufrêne YF, Gomelsky M, Moroni A, Ramstedt M, Rumbaugh KP, Schulte T, Sun L, Åkerlund B, Römling U. Biofilm formation – what we can learn from recent developments. J Intern Med. 2018 Oct;284(4):332-345. doi: 10.1111/joim.12782. Epub 2018 Jul 9. PMID: 29856510; PMCID: PMC6927207.
Dinicola, S et al. “N-acetylcysteine as powerful molecule to destroy bacterial biofilms. A systematic review.” European review for medical and pharmacological sciences vol. 18,19 (2014): 2942-8.
Feng, Jie et al. “Identification of Essential Oils with Strong Activity against Stationary Phase Borrelia burgdorferi.” Antibiotics (Basel, Switzerland) vol. 7,4 89. 16 Oct. 2018, doi:10.3390/antibiotics7040089
Halstead, Fenella D et al. “The Antibacterial Activity of Acetic Acid against Biofilm-Producing Pathogens of Relevance to Burns Patients.” PloS one vol. 10,9 e0136190. 9 Sep. 2015, doi:10.1371/journal.pone.0136190
Huang, Ruijie et al. “Bacterial interactions in dental biofilm.” Virulence vol. 2,5 (2011): 435-44. doi:10.4161/viru.2.5.16140
Lopez-Nguyen Darrene, Badet Cecile, “Experimental Models of Oral Biofilms Developed on Inert Substrates: A Review of the Literature”, BioMed Research International, vol. 2016, Article ID 7461047, 8 pages, 2016. https://doi.org/10.1155/2016/7461047
Mohsenipour, Zeinab, and Mehdi Hassanshahian. “The Effects of Allium sativum Extracts on Biofilm Formation and Activities of Six Pathogenic Bacteria.” Jundishapur journal of microbiology vol. 8,8 e18971. 25 Aug. 2015, doi:10.5812/jjm.18971v2
Nawrocka, Agnieszka, and Monika Łukomska-Szymańska. “Extracted human teeth and their utility in dental research. Recommendations on proper preservation: A literature review.” Dental and medical problems vol. 56,2 (2019): 185-190. doi:10.17219/dmp/105252
Seneviratne, Chaminda Jayampath et al. “Dental plaque biofilm in oral health and disease.” The Chinese journal of dental research : the official journal of the Scientific Section of the Chinese Stomatological Association (CSA) vol. 14,2 (2011): 87-94.