Nina Peloso, Year 2 Engineering
Abstract
While more than one hundred thousand annual deaths are attributed to drownings worldwide, Shallow Water Blackout (SWB), a leading subset, is often overlooked. Thus far, efforts have largely consisted of awareness organizations and small-scale campaigns; although the feasibility of a wearable device has been explored in the prevention of freediving blackout, the applicability to pool-wear remains limited. This paper looks at the possibility of utilizing a wearable MAX30100-based circuit to provide a real-time alert when SWB occurs. The circuit incorporated an LED ‘alert’ and was based in an Arduino nano; but was not completed due to obstacles in i.c. design. Further work must be done to investigate the viability of a real-time alert in the detection of SWB using a circuit of common products to ensure a low-cost and the achievability of commercial use.
Introduction
Shallow Water Blackout (SWB) describes the loss of consciousness underwater via brain hypoxia. During the event, the body’s co2 levels drop, often as a result of mild hyperventilating which begets the narrowing of the cerebral blood vessels and impairs the swimmer’s inhibition to breathe; spurring a rapid fall-off in blood oxygen saturation (hypoxia), and unconsciousness. (Swimming Canada, “Shallow Water Blackout (SWB)”). According to Swimming Canada, of the more than 140,000 annual deaths due to accidental drownings worldwide, SWB cases account for roughly 20% (Swimming Canada, “Shallow Water Blackout (SWB)”). So far, much of the effort around reducing SWB has focused on raising awareness and ameliorating the risks through encouraging ‘safe swimming behaviors’ such as swimming with a partner, avoiding overexertion, and refraining from breath-holding for extended periods of time (Shallow Water Blackout Prevention, “How it Happens”). Elite and novice swimmers find themselves at the crossroads of these risk factors, and must be kept in mind when measuring the efficacy of preventative devices.

Figure 1: Freediver protective vest supporting a diver (Maas, “The Case for a Device to Manage Freediving Blackout”)
Presently, one device exists in the form of a “freediver protective vest” (Figure 1) (Maas, “The Case for a Device to Manage Freediving Blackout”). The vest uses depth and time sensors to detect when a diver is too deep or has been underwater for longer than the time set-up by the diver, at which point the vest inflates and pulls them towards the surface. However, one problem with the device is the lack of applicability to pool drownings, where a bulk of SWB cases occur (Boyd et al., 2015; in such shallow water the depth counter is rendered ineffective. Finally, relying on the time counter is especially risky given than SWB drownings are shown to induce death and brain damage in a fraction of the time of normal drownings, where 6-8 minutes might become 2-3 (Bart & Lau, 2021) (Bowman, “Shallow Water Blackout) (Boyd et al., 2015). When a time counter is utilized, thresholds can be difficult to gauge, as tolerances often depend on the athlete, individual, or even specific occurance. The aim of this project is to investigate a method of creating a arduino-based wearable device which mitigates these issues, with the ability to produce a real-time alert if the wearer experiences oxygen/and or blood co2 deprivation levels that put them at a risk of blacking out, thus reducing the propensity for SWB in novice and elite swimmers.
Materials & Methods
The circuit was structured around an integrated pulse oximetry and heart-rate unit, the MAX30100 (Walfront/Amazon). In this case, pulse oximetry records a wearer’s ‘SpO2’, or the percentage of oxygen-carrying hemoglobin present in the bloodstream divided over the total functional hemoglobin in the patient’s bloodstream (Mayo Clinic, 2018) called peripheral oxygen saturation, similar to SaO2 (or arterial oxygen saturation), which takes into account the functional and the non-functional hemoglobin (Hafen & Sharma, 2021)(Mayo Clinic, 2018). The instrument calculates an SpO2 reading by calculating the differential between the red/infrared light received after passing through the wearer’s extremity from a corresponding light emitting diode (LED) situated within each sensor (Philips Medical Systems, 2016); this is possible as oxygenated hemoglobin absorbs more infrared light than red light while deoxygenated hemoglobin responds inversely (University of Iowa, 2017.) Although much of the emittance is absorbed by bone, soft tissue and venous blood, the oscillating values of arterial blood (due to pulsation) (Philips Medical Systems, 2016) allows a measurement that may be used in the detection of peripheral oxygen saturation, and in tissue hypoxia by extension (Hafen & Sharma, 2021)(Philips Medical Systems, 2016).
Using this mechanism, the circuit incorporated a MAX30100 integrated circuit, an LED, and three 470 Ω resistors for use with the Arduino nano (Arduino), selected with wearability in mind. Pictured in Figure 2, 3, are the circuit and schematic respectively (modeled in TinkerCAD). In the schematic/model, the MAX30100 is an 8-pin header denoted with the notes tool while an Arduino uno was used in place of an Arduino nano due to the limited selection of TinkerCAD components. However, in the case of the latter, there is no meaningful difference in this instance as they are identical in terms of computational capacity in the hardware and peripherals, given that they both rely on the reduced instruction set computer (RISC) ATMEGA328P-NP microcontroller (Atmel, “ATMEGA328P Datasheet”).
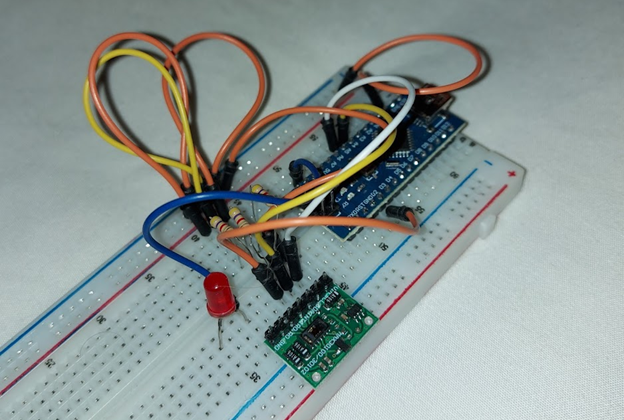
Figure 2: Photograph of the designed circuit/prototype
In accordance with the processor (ATMEGA328P-NP), the MAX30100 module operates on a I2C interface, a protocol for sending and receiving data, also called “two-wire protocol”. Specifically, the circuit utilizes the serial clock-pin (SCL) to send ‘bits’ of information at set intervals to the i.c.’s serial data-pin (SDA) which decodes the resultant sequence of bits (Zambetti, 2022). On the Arduino nano, analog pin 4 and 5 may function as the data and clock lines respectively (Dannegard, 2022), which can be achieved through the use of the “Wire.h” library shown to preface the program in Figure 4. Coupled with the “MAX30100_PulseOximeter.h” library, the program calls upon the SCL and SDA pins to read the values of red/infrared light perceived by the i.c. periodically. Thus, the SpO2 and heart rate is meant to be read by the device, in future iterations the SpO2 reading may be converted to a float value and made to dictate a conditional which controls the LED, or the ‘alert’; that is, if SpO2 % > 95 % (or ± 0.10) (as per medical convention (Mayo Clinic, 2018)), then “digitalWrite (3, HIGH)” where digital-pin ‘3’ functions as the output for the LED. The modified source-code (How To Electronics, “Interfacing MAX30100 Pulse Oximeter Sensor with Arduino”) uses a midrange ‘reporting period’ of 1000 milliseconds, which denotes the rate at which the period timing occurs, characteristic to I2C protocol.
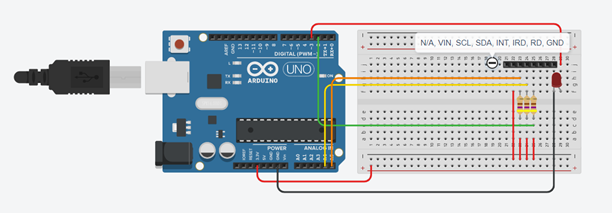
Figure 3: Schematic of design, modeled in TinkerCAD.
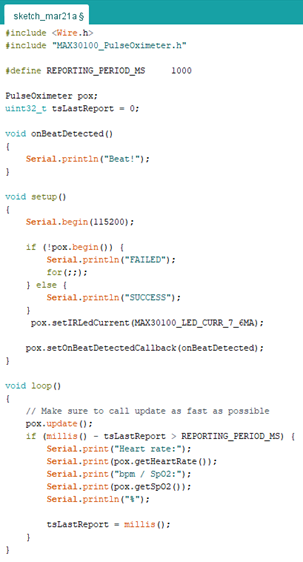
Figure 4: Modified source-code of the device (How To Electronics, “InterfacingMAX30100 Pulse Oximeter Sensor with Arduino”)
However, the resultant circuit encountered impassable issues in the internal functionality of the i.c. module; efforts to amend the design are present in the external resistors (470 Ω).
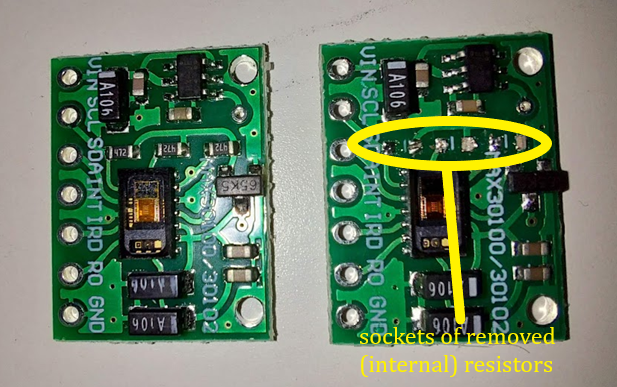
Figure 5: The MAX30100 i.c. was modified in an attempt to assuage voltage disparities, (left) the unmodified module versus the module with internal resistors removed (right) to be relocated externally, onto the circuit.
Results
Although the initial aim was to investigate a method of developing a wearable device capable of real-time alert for SWB cases; no tangible device was produced. Since the original MAX30100 i.c. includes a trio of 470 Ω resistors in parallel, the i.c.’s voltage regulators ensure a 1.8 V voltage through each, and thus they are unable to pull-up or down due to incompatibility with the 3.3 V of the ATMEGA328 microcontroller of the Arduino nano. (See Figure 5) to mitigate the issue, the module’s internal resistors were unsoldered, and three 470 Ω resistors were instead incorporated into the (external) circuit design. However, this design yielded no difference to the functionality of the module. Rather, the design and background provide a direction of research for future development in the mitigation of SWB through wearable means, as well as a survey of potential obstacles when producing a MAX30100-based device.
Discussion
Pulse oximeter integrated circuit devices akin to, or at the production level of, theMAX30100 are desirable in compactness, availability and price point. However, usability is a crucial factor in developing a medical device with the goal of real-time alert; thus, this design may act as a point of reference for future prototypes in the interest of realtime alerts using the MAX30100, or those that target SWB alerts through arduino-based devices.
Future research could develop the concept of a wearable arduino-based device through the utilization of a commercial pulse oximeter’s internal mechanism; and therein optimizing the device with SWB in mind. Prioritizing not only the accuracy and speed of the alert, but comfortability/sleekness of the device, positioning of sensors/emitters and
waterproofness.
Since pulse oximeters work by emitting through rad/infrared LEDs from one side and receiving on the other, extremities are ideal, the earlobe and forehead are also commonly used (Pantelopoulos & Bourbakis, 2010). Of these places, fingers are usually selected for the ease and acceptable accuracy (Pantelopoulos & Bourbakis, 2010). However, for the application to SWB detection, fingers are undesirable in part because they are instrumental to stroke and hence form during swimming, which is important to maintain given the target audience of elite and novice swimmers. Additionally, since SWB is resultant of cerebral hypoxia, the measurement of hypoxia in extremities may not be as accurate as a closer probe-location (Pantelopoulos & Bourbakis, 2010) (Seifi, et al., 2018). With these issues in mind, studies on the differing probe-locations for SpO2 detection such as Seifi, et al. (2018), found earlobe-probes to be the most accurate in these measurements (Seifi, et al., 2018). A conclusion which is endorsed in hypothermia patients, (Simon & Clark, 2002) patients under anesthesia, (Eberhard, et al., 2002) as well as in the detection of apnea (Lindholm, et al., 2007). In the lattermost case, the superiority of earlobe-probes were attributed to abilities in detecting ‘peripheral hypoxia’, a loss of oxygen in tissues adjacent or beside the site of the probe.
A successful design could have these considerations in mind to produce a more optimal wearable-device which scans the earlobe for SpO2 deprivation, is comfortable and relies on the principles of SWB drownings discussed in this paper.
References
Atmel. (n.d.). 8-bit AVR Microcontroller with 32K Bytes In-System Programmable Flash. Atmel. Retrieved from
https://ww1.microchip.com/downloads/en/DeviceDoc/Atmel-7810-AutomotiveMicrocontrollers-ATmega328P_Datasheet.pdf
Bart, R. M., Lau, H. (August 1, 2021). Shallow Water Blackout. StatPearls [Internet]. NCBI. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK554620/
Bowman, B. (n.d.). Shallow Water Blackout. USA Swimming. Retrieved from
https://www.usaswimming.org/docs/default-source/sportsciencedocuments/shallow_water_blackout_asca.pdf
Boyd, C., Levy, A., McProud, T., Huang, L., Raneses, E., Olson, C., & Centers for Disease Control and Prevention (May 22, 2015). Fatal and Nonfatal Drowning Outcomes Related to Dangerous Underwater Breath-Holding Behaviors — New York State, 1988–2011. MMWR. Morbidity and Mortality Weekly Report. vol. 64(19). pp. 518-521. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4584570/
Dannegard, Benjamin. (April 11, 2022). Connecting Two Nano Every Boards Through I2C. Arduino. Retrieved from https://docs.arduino.cc/tutorials/nano-every/I2C
Eberhard, P., Gisiger, P. A., Gardaz, J. P., & Spahn, D. R. (2002). Combining
transcutaneous blood gas measurement and pulse oximetry. Anesthesia and
analgesia, 94(1 Suppl), S76–S80.
Hafen BB, Sharma S. Oxygen Saturation. [Updated 2021 Aug 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK525974/
How To Electronics. (August 3, 2021). Interfacing MAX30100 Pulse Oximeter Sensor with Arduino. How To Electronics. Retrieved from
https://how2electronics.com/interfacing-max30100-pulse-oximeter-sensorarduino/
Lindholm, P., Blogg, S. L., & Gennser, M. (2007). Pulse oximetry to detect hypoxemia during apnea: comparison of finger and ear probes. Aviation, space, and environmental medicine, 78(8), 770–773.
Maas, T. (n.d.). The Case for a Device to Manage Free Diver Blackout. Retrieved from
http://rockymountainspearfishing.org/articles/THE_CASE_FOR_A_DEVICE_TO_MANAGE_FREEDIVER_BLACKOUT.pdf
Mayo Clinic. (December 1, 2018). Hypoxemia (low blood oxygen). Mayo Foundation for Medical Education and Research (MFMER). Retrieved from
https://www.mayoclinic.org/symptoms/hypoxemia/basics/definition/sym-
20050930
Pantelopoulos, A., & Bourbakis, N.G. (2010). A Survey on Wearable Sensor-Based Systems for Health Monitoring and Prognosis. IEEE Transactions on Systems, Man, and Cybernetics, Part C (Applications and Reviews), 40, 1-12.
Philips Medical Systems. (n.d.). Understanding Pulse Oximetry SpO2 Concepts. Philips. Retrieved from
https://www.documents.philips.com/doclib/enc/fetch/586262/586457/Understanding_Pulse_Oximetry.pdf
Seifi, S., Khatony, A., Moradi, G. et al. (2018) Accuracy of pulse oximetry in detection of oxygen saturation in patients admitted to the intensive care unit of heart surgery: comparison of finger, toe, forehead and earlobe probes. BMC Nursing. 17, 15. https://doi.org/10.1186/s12912-018-0283-1
Simon, B. Stephen, Clark, A. Robyn. (2002) Using pulse oximetry: a review of pulse oximetry use in acute care medical wards. Clinical Effectiveness in Nursing. 6(3-4). 106-110. https://doi.org/10.1016/S1361-9004(02)00088-2.
Swimming Canada (n.d.). Shallow Water Blackout (SWB). Swimming Canada. Retrieved from https://www.swimming.ca/en/safe-sport/education/mental-andphysical-health/shallow-water-blackout-swb/
Iowa Head and Neck Protocols. (October 3, 2017). Pulse Oximetry Basic Principles and interpretation. University of Iowa Health Care. Retrieved from https://medicine.uiowa.edu/iowaprotocols/pulse-oximetry-basic-principles-andinterpretation#:~:text=Oxygenated%20hemoglobin%20absorbs%20more%20infrared,and%20absorbs%20more%20red%20light.
Zambetti, Nicholas. (April 11, 2022). A Guide to Arduino & The I2C Protocol (Two Wire). Arduino. Retrieved from https://docs.arduino.cc/learn/communication/wire