Felix Zhou, Year 2 Research
Abstract
Ocean acidification is an increasing problem for the world’s aquatic ecosystems as carbon dioxide emissions increase, particularly affecting calcifying organisms such as shellfish. Since such organisms are vital to the health of our ecosystems, it is highly important to understand exactly how shellfish would be affected in the future by decreasing pH in ocean water. This study found that in solutions simulating both current ocean pH and projected ocean pH in about a century’s time, significant weakening and dissolution of clam shells occurred. However, these results are insufficient to form solid conclusions on what to expect in the coming decades as there was little difference between the control and acidified samples observed, arising from a number of factors including the limitations of working with household materials. Despite the unexpected result and uncertainties, the fragility of the shells post-submersion may serve as an indicator of the impact even relatively small changes in pH can have on marine life, though further research will be needed to assess this issue.
Introduction
Ocean acidification is a worsening problem as the oceans absorb more carbon, setting off reactions that lower the pH levels of ocean water (“What is Ocean Acidification”). The pH levels of surface ocean waters have decreased in pH by about 0.1 since the beginning of the Industrial Revolution (“What is Ocean Acidification”), which represents an increase in acidity of approximately 30% (Dupont and Pörtner, 2013), and which could double or triple still by the year 2100 (Dupont and Pörtner, 2013), and to as low as 7.7 in the most extreme scenario (Jiang et al, 2019). This can have dramatic consequences for calcifying species such as oysters, sea urchins, and corals(“What is Ocean Acidification”), as falling pH levels decrease environmental calcium carbonate and impact those organisms that use this material to build shells or skeletons (“What is Ocean Acidification”). Existing research reveals such negative effects as catastrophic die-offs of wild oyster stocks on the American west coast (Dupont and Pörtner, 2013) and increased erosion to damage to coral reefs (“What is Ocean Acidification”). Therefore, having a clear understanding of the potential implications and consequences of such a significant change in pH is supremely important.
This experiment hopes to do additional research on the effects of further acidification, focusing on calcifying marine organisms in cold and temperate waters. Specifically, it will be studying the effects of lowering the pH level in a business-as-usual scenario on the size and durability of clam shells, and comparing the experience of shells at current and acidified pH levels, in order to study the extent to which such organisms could be impacted in the near future. This will be done by collecting several relatively intact shells off local beaches and placing some in a container filled with salt water held at the current ocean pH level, which will serve as a control sample. The remaining shells will be placed in acidified water with a pH level as predicted in a business-as-usual scenario.
Materials and Methods
A number of relatively intact and recently washed up seashells from local shellfish were collected off Spanish Banks Beach in Vancouver Canada, at low tide. The shells were kept in a closed, dry container in a dark, cool room until they were ready to be placed in solution. Three to four shells of similar appearance were selected to be placed in each of the acidified and control solution. Immediately before placing into solution, measurements were made of each shell’s dimensions at its longest point and widest point. Qualitative observations, specifically texture and colour, were also noted.

Figure 1: Set up of shells in container, side view.

Figure 2: Set up, top view.
Two 1L samples of water were prepared (See Figures 1 & 2). Originally, the experiment was to use 2 identical 1L glass beakers; however, one of the beakers unexpectedly suffered a breakage, and was therefore substituted for a stainless steel container, with the amount of water measured out separately and then transferred. The type of container used does not seem to effect the results of the experiment, as both containers contained the same amount of water, at a high enough level to completely submerge every shell with room left over, and both containers were kept closed for the duration of the experiment except when measurements were being taken. Neither container appeared to contain materials that could potentially dissolve into the water in a significant enough quantity to noticeably affect the measurements. 20 to 25 grams of sea salt were then added to each sample of water, to simulate the salinity of the local seawater in the area around Vancouver. Then, varying amounts of sodium bicarbonate and acetic acid solution were added to each sample of water until the desired pH level–7.6 for the acidified sample, 8.1 for the control sample– was obtained as measured by a digital pH meter. A lower value than the 2100 projection of 7.7–7.8 was used as this study sought to explore the long term impacts of acidification many decades into the future, and it is reasonable to expect that acidification will continue well past 2100, and therefore values further into the future would be more helpful in evaluating the possible extent of the damage caused. The pH value read was then checked against a strip of pH indicator paper to ensure general accuracy. After the solutions were prepared, the shells were placed into solution, and the containers covered and refrigerated at a constant temperature of approximately 4 degrees Celsius, to simulate the cold water environment of local oceans, though the temperature did briefly rise by a few degrees on when the samples were removed from the refrigerator for periodic measurements and corrections of the pH level. After two weeks, with occasional measurements and corrections of pH level in the meantime, the shells were removed from the solution. The same measurements of length and width, texture, and colour taken at the beginning of the experiment were taken again, and the data compared to check for trends as well as differences between the acidified and control samples.
Results
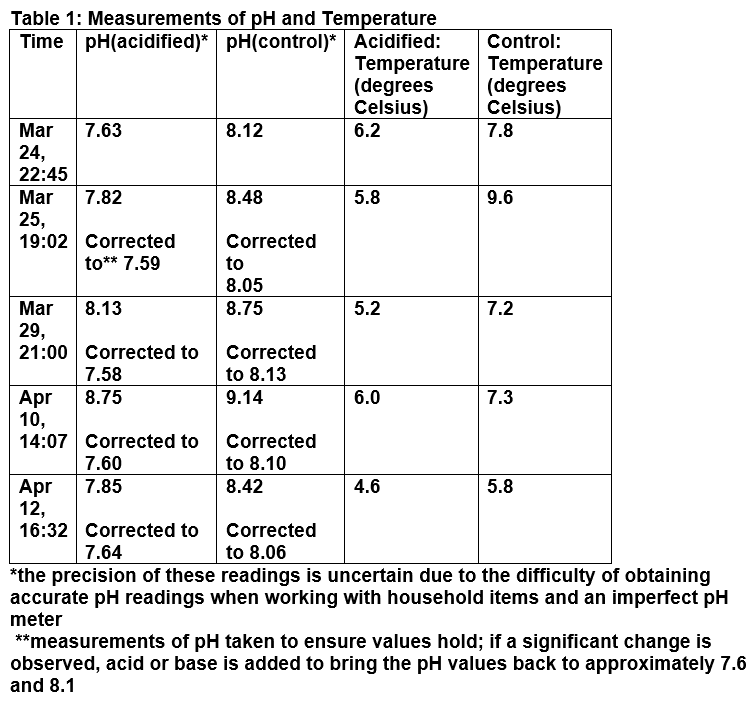
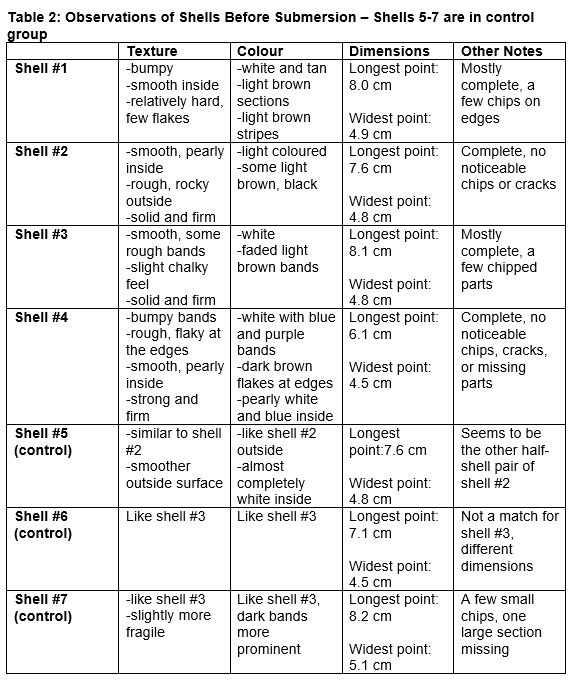
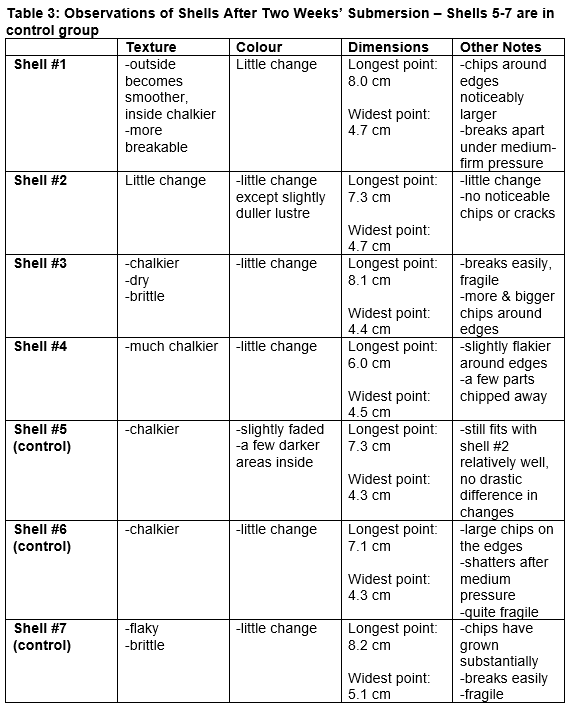
Based on the measurements made and observations taken, the dimensions of each shell seem to have decreased slightly after two weeks in both the control and acidified samples; some shells exhibited no measurable changes, while other shells had their dimensions decreased by up to a few millimetres. In general, the width of the shells exhibited a greater amount of decrease than the length; however, this does demonstrate that some dissolution has occurred. Moreover, the shells post-submersion appeared to have larger chips and more missing parts visible to the naked eye than they did pre-submersion. Colour did not seem to be significantly affected in either the control or acidified samples. Post-submersion, the shells in both groups were chalkier, and some also became more brittle and fragile to the touch; some shattered easily (Figure 3 & 4). There were few differences in the changes observed between the control and acidified groups. Additionally, over the course of the experiment, the pH of both samples of water consistently increased considerably over time, and required regular correction, though they were held at a relatively constant temperature.

Figure 3: Shell #6 pre submersion

Figure 4: shell #6, post-submersion
Discussion
The results indicate that some dissolution did occur in both samples. The dimensions, especially the width, shrank by up to a few millimetres after two weeks of submersion. The enlarged chips and missing parts are also evidence of substantial dissolution of the shells. The constantly rising pH level may be due to carbonate from the shells dissolving into solution in a relatively small amount of water; indeed, at several points in the experiment residue resembling a precipitate was found in the bottom of both containers. This precipitate may be some carbonate compound originating from the shells, perhaps forming a precipitate with some trace minerals in the water. The acid used, acetic acid, was already dissolved when added to the water and the base used, sodium bicarbonate, has a high solubility in water and was ensured to have been thoroughly dissolved into solution each time. Thus, the above, in addition to the chalky and brittle texture of the shells post-submersion, and the fact that the shells were much more easily broken than they were before the experiment, is strong evidence pointing in favour of some dissolution having occurred.
Interestingly, however, there did not appear to be a measurable difference between the acidified and control samples – both exhibited strikingly similar changes. This is unexpected as water held at the pH of current ocean water should not have dissolved shells quite as quickly has more acidified water. In fact, the extent of the dissolution is surprising, given that the constantly rising pH level in both samples of water would have caused the shells to have spent a considerable amount of time in basic water. These results conflict with a very similar study (McClintock et al, 2009) that showed significantly higher dissolution rates in acidified water. Bednaršek et al. (2014) also demonstrated a strong positive correlation between severe shell damage and acidification. Since multiple previous studies have come to the different conclusion that acidified water does indeed cause significantly more damage to shellfish, it is quite probable that another unknown variable or variables may be the main driving force of the dissolution rather than the effects of acidification as previously expected; however, it is currently not known what these variables may be, if they exist. More experimentation will be needed to study the effects of different variables in isolation and find suitable candidates. Therefore, further studies will have to be done in order to discover the reason behind these results, as well as to truly understand what to expect in the event of a business-as-usual acidifying of our oceans.
There is great uncertainty in the quantitative measurements in this study. The pH meter’s readings were not always completely accurate, even after calibration. Given that this experiment was performed in an at-home environment, with mostly household supplies and limited access to equipment, there is a limit on the precision of the measurements as well as on how many variables could be considered in fill. Therefore, similar experiments may have to be repeated in the future to obtain more precise results and give consideration other underlying factors, including how much of the dissolution can be specifically attributed to lower pH water.
Future experiments may use the same number of shells in a much larger quantity of water to consider the reason behind the pH rise and whether the phenomenon is connected to the dissolution of the shells. Future studies may also want to conduct the experiment over several different lengths of time to determine how shell dissolution varies with time, and whether over a longer period of time there may appear a greater difference between the two samples. Using live samples in future experiments may also paint a clearer picture of how acidification will affect living organisms in the real world, and be able to take into greater consideration the complex chemical interactions between organisms and their environment, possibly allowing many more variables to be considered than could be by studying only shells. In any case, it is imperative to understand as well as possible how ocean acidification will impact calcifying marine organisms and, by extension, the entire marine ecosystem, in order to plan scientifically sound policies to address these changes.
References
Bednaršek, N., et al. “Limacina Helicina Shell Dissolution as an Indicator of Declining Habitat Suitability Owing to Ocean Acidification in the California Current Ecosystem.” Proceedings of the Royal Society B: Biological Sciences, vol. 281, no. 1785, 2014, p. 20140123., doi:10.1098/rspb.2014.0123
Dupont, S., Pörtner, H. “A snapshot of ocean acidification research.” Marine Biology, vol. 160, 2013, pp. 1765-1771 https://doi.org/10.1007/s00227-013-2282-9
Jiang, LQ., Carter, B.R., Feely, R.A. et al. “Surface ocean pH and buffer capacity: past, present and future.” Sci Rep, vol. 9, no. 18624, 2019, https://doi.org/10.1038/s41598-019-55039-4
McClintock, James B., et al. “Rapid Dissolution of Shells of Weakly Calcified Antarctic Benthic Macroorganisms Indicates High Vulnerability to Ocean Acidification.” Antarctic Science, vol. 21, no. 5, 2009, pp. 449–456., doi:10.1017/s0954102009990198
“What Is Ocean Acidification?” PMEL Carbon Program. (n.d.)https://www.pmel.noaa.gov/co2/story/What+is+Ocean+Acidification%3F