Daniel Kim, Year 2 Engineering
Abstract
Chemical pollution is a growing issue that prevents access to clean freshwater. Inspired by this issue, this project aims to investigate the efficiency of different natural filters. The two filters that were explored in this investigation were ground coffee beans and charcoal grounds. To test the filtration capabilities, a 0.10 M calcium chloride solution was poured through either the coffee grounds or charcoal through a prepared apparatus. The filtered calcium chloride solution was then precipitated using a 0.10 M sodium carbonate solution. The precipitate was then dried and measured. This project found that the ground charcoal was the best filter between the two options, however, it did not yield significant results, and more improvements and experimentation must be made before further conclusions are formed.
Introduction
The pollution of water due to chemicals has become an increasingly prevalent issue and is responsible for the sickness of approximately one billion people (Denchak, 2018). Among the various types of pollutants in the water, chemicals and heavy metals can pose serious health ramifications for both the ecosystem and for humans that access polluted water (Denchak, 2018). When the pH in water decreases (due to natural or human-based causes), the solubility of the metals in the water increases and the metal particles cause the water to become toxic (McNamara 2017). Consequently, it has become imperative to find ways to filter said chemicals from water sources. As a result, this project investigates the different two potential filters: coffee grounds and charcoal and whether increasing their mass would impact their filtration capabilities. To further clarify, this project attempts to answer the research question:
Are ground coffee and ground charcoal viable filters of chemically polluted water?
Due to safety constraints, chemicals including heavy metals were not available. Thus, calcium chloride was utilized to emulate. Calcium chloride is another pollutant which impacts freshwater sources when entering freshwater systems (Hintz and Relyea, 2017). The inspiration for this project came after hearing about a local community lead poisoning incident in my community, leading me to research about the potential solutions for this issue, culminating in my interest.
Theory
Ground charcoal was chosen as activated charcoal filters. They are commonly used and an efficient natural filter. The chemicals in the water are attracted to the carbon in the charcoal and readily bonds to these impurities (McNamara, 2017). Activated charcoal is defined as charcoal treated with oxygen to create millions of miniscule pores between the carbon atoms. The charcoal acts as an adsorbent, which is defined to be the process where substances adhere to the adsorbent’s surface by chemical attraction (Peshin, 2019). This concept of adsorption is different than that of liquid absorption as absorption involves the process in which substances are vacuumed in an adsorbent’s material whereas adsorption merely includes the adhering of the substance (Steele, 2017). This difference is further illustrated in Figure 1.
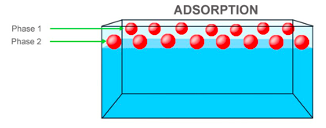
Figure 1a): Illustration of Adsorption
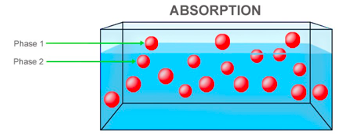
Figure 1b): Illustration of Absorption (Peshin, 2019)
Furthermore, due to the great number of pores, the charcoal has a very large surface area, giving it many bonding sites (Peshin, 2019). In the physical adsorption process itself, the molecules that are to be adsorbed are held together by the carbon’s surface by the weak intermolecular forces known as Van der Waal’s forces and as a result, the activated charcoal does not change the solution on a chemical basis (Steele, 2017). This adsorption process for activated charcoal is illustrated in Figure 2.
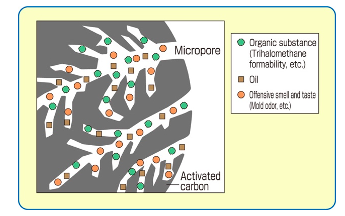
Figure 2: Illustration of activated charcoal adsorption on molecular basis (Steele, 2017)
While activated charcoal was not utilized in this experiment due to limited availability of pure activated charcoal for commercial sale, ground charcoal was utilized in the hopes of emulating the same effect as the activated charcoal. However, this is a component that is necessary to keep in mind as this use of a different form of charcoal is not the same as the activated charcoal and thus the properties cannot be expected to be the same and must be considered when examining the results. The coffee grounds were selected as past studies have shown that coffee grounds can act as adsorbents for heavy metals (Moelyaningrum et al, 2018).They concluded through their studies that the Robusta coffee adsorbed some of the lead particles in the solution which reduced the lead concentration of the water (Moelyaningrum et al, 2018). A similar technique was utilized in a 2016 study by researchers at Istituto Italiano di Tecnologia. They created bio elastomeric composite foams to remove heavy metal ions such as lead and mercury from water (Chavan et al, 2016). While this investigation cannot follow the specific procedures of these aforementioned scientific research papers, the findings from the research serve as the scientific basis and reasoning behind the selection of coffee grounds for this project.To test the filtration capabilities of these two filters, a solution of calcium chloride, an ionic compound that is soluble in water, was prepared to see if the filters could adsorb the ions in the solution. After being poured through the filter, the remaining solution was then precipitated by pouring a sodium carbonate solution in excess, which would form an insoluble salt when reacting with the calcium chloride. This is showcased in the following equation.
Na2CO3(aq)+ CaCl2(aq)→CaCO3 (s)+ 2 NaCl (aq) (eq.1)
If the filters could filter some ions from the solution, it would result in fewer ions being present in the filtered solution which would result in less mass of precipitate being formed as there are fewer ions in the limiting reagent.
Materials and Methods
The coffee grounds were obtained from home-stored coffee grounds (Starbucks) and commercial charcoal (Royal Oak) was obtained through Home Depot and ground into smaller portions using a mortar and pestle.

Figure 3: Materials and Apparatus
A general filtration apparatus was created in order to ensure consistency in between trials and to test the feasibility of the water filtration mechanism that was created. This water filtration apparatus was created using a round plastic container approximately 20 cm high and 13 cm wide (Dollar Store) and a 8 cm long and 15 cm wide circular funnel (Dollar Store) that led to the plastic container’s opening. A circular funnel was chosen so if the solution were to land on the container, it would be directed towards the filter. A4 cm wide and 2 cm high round sift (Dollar Store) was placed inside the circular funnel to hold the ground charcoal and coffee grounds in place for the solution to be poured through. The ground charcoal and coffee grounds were spread as uniformly and consistently as possible between each trial. A 250mL beaker was then inserted inside the plastic container and underneath the funnel as a means of capturing the filtered solution. This is shown in Figure 4 and 5.

Figure 4: Annotated Picture of Apparatus

Figure 5: Annotated picture of inside the apparatus
The CaCl2 solution was prepared by using an electronic balance (ANDBalances) to weigh out 5.55g of the CaCl2. Then, the 5.55 g CaCl2 was then inserted into 500 mL of water that was measured in advance using a graduated cylinder, forming a 0.10M solution. The Na2CO3 solution was prepared by putting in 5.31g of the Na2CO3 which was the inserted in 500 mL in a similar fashion, thus forming a 0.10M solution. In order to test the filtration capacity of the charcoal and the coffee grounds, the charcoal and coffee grounds were weighed and inserted into the sift as shown in Figure 4. To see whether or not increasing the mass of the filter would increase the filtration capacity, the range of masses of the respective filters for the data collected varied from 1.00 –4.00 g, with increasing intervals of 1.00 g. This was then repeated 3 times per different individual value of mass. For each individual trial, 10.0 mL of the CaCl2solution was measured using a graduated cylinder and poured through the sift and filter. After being poured through the sift and filter, the filtered solution was then precipitated using 15.0 mL of the Na2CO3 solution.The precipitated solution was then poured through a ceramic funnel that was inserted into an Erlenmeyer flask. A filter paper was placed on the inside of the ceramic funnel to filter out the solution yet keep the solid precipitate. To measure the filtration capability of the different filter, the mass of the precipitate is weighed and compared to the theoretical yield. The mass of the precipitate is measured by weighing the total mass of the Erlenmeyer flask, the ceramic and the filter paper prior to pouring the solution through the Erlenmeyer flask and then weighing it afterwards after pouring out the liquid solution from the Erlenmeyer flask and leaving the precipitate to dry so that there is no water in the precipitate and then calculating the difference between the final mass of the system containing precipitate and the system before.
Results
The following data was measured and recorded in Data Table 1.
Table 1. Raw data
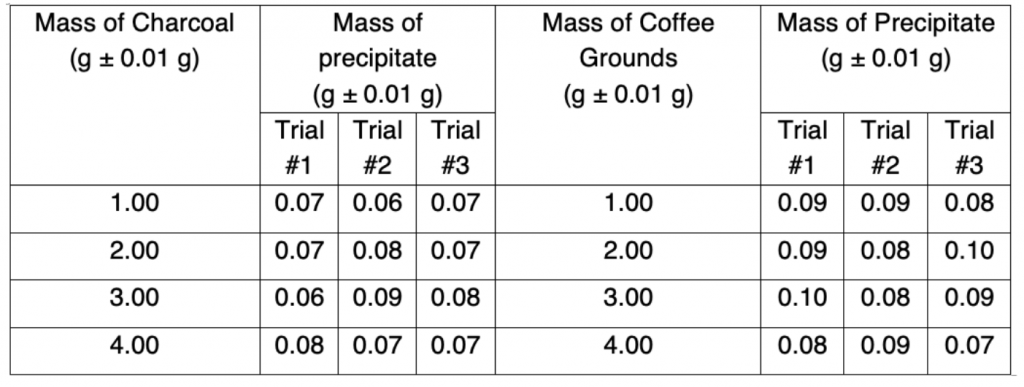
This data is now organized in the processed data table:
Table 2: Processed Data
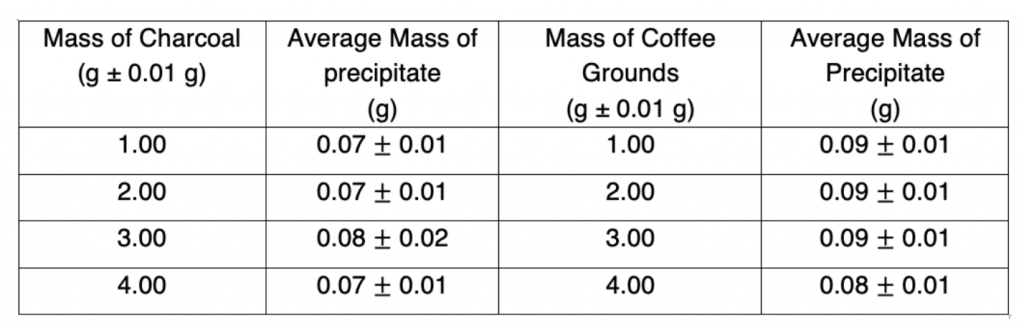
This is now plotted with the mass of charcoal/coffee grounds on the x-axis as it is the independent variable and the average mass of precipitate on the y-axis.
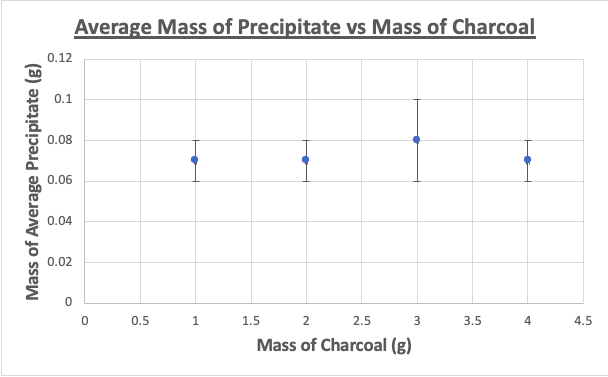
Figure 6: Average mass of precipitate vs Mass of Charcoal Graph
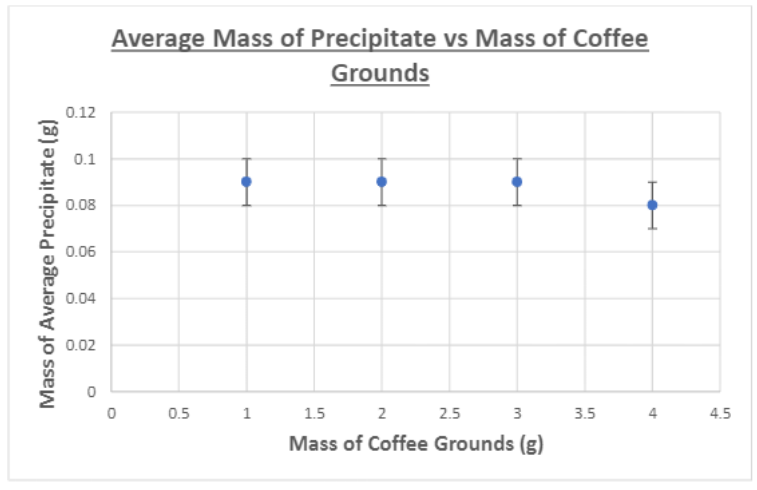
Figure 7: Average mass of precipitate vs mass of coffee grounds
Discussion
From the results, it can be seen that there was no relationship between increasing the mass of the filters and the amount of precipitate as the mass of the precipitate did not decrease after increasing the mass of both the charcoal and the coffee grounds. Furthermore, there was no line of best fit drawn due to there being minimal amounts of data. The data showcases that the coffee grounds reduced the amount of precipitate by an average of 0.01gcompared to the theoretical value whereas the charcoal reduced the amount of precipitate of an average of 0.03g. Thus, compared relatively to one another, the amount of precipitate when filtered by the charcoal is less than that of the coffee grounds and this could potentially be explained by the greater adsorbent properties of the charcoal. However, seeing as this is not activated charcoal, the absorbency of the charcoal in this experiment would have significantly worse adsorbent capabilities. However, it is important to note that although they were tested at the same mass, the coffee grounds filled a significantly smaller area within the sift compared to the charcoal, which indicates that a potential explanation of the results is that the calcium chloride solution simply came in contact with a smaller area of coffee grounds (although its surface area is much larger)as some of the solution passed straight through the sift instead of fully coming in contact with the filter. However, the apparatus was the fault as some of the solution did not pass entirely through the filter in the sift, introducing a massive source of error in the apparatus. Ultimately, it can be concluded that, in the frame of this experiment, the charcoal and the coffee grounds did not serve as adequate filters and yielded minimal results. One key improvement that can be implemented would be to use a higher concentration of solution and to pour more solution through the filters so the results would have greater values of the mass of precipitate to analyze. Furthermore, another area for improvement would lie in using other chemicals and materials. Seeing that this experiment served as an attempt to emulate the science behind activated charcoal filter and coffee ground composite foams to filter heavy metals, using the chemicals and materials outlined in Moelyaningrum and Chavan’s experiments would provide improved results. While this experiment did not show promising results, it can be noted that more data must be collected and an apparatus with a less source of error must be utilized in order to fully conclude these findings.
References
Chavan, Asmita et al. “Spent Coffee Bioelastomeric Composite Foams for the Removal of Pb2+and Hg2+from Water” 1 Sep 2016. ACS Sustainable Chem. Eng.2016, 4, 10, 5495–5502.doi.org/10.1021/acssuschemeng.6b01098
Denchak, Melissa. “Water Pollution: Everything You Need to Know.” NRDC, NRDC, 14 May 2018, www.nrdc.org/stories/water-pollution-everything-you-need-know.
Hintz, William D, and Rick A Relyea. “Impacts of road deicing salts on the early-life growth and development of a stream salmonid: Salt type matters.”Environmental pollution (Barking, Essex : 1987)vol. 223 (2017): 409-415. doi:10.1016/j.envpol.2017.01.040
McNamara, Phil. “5 Benefits of Using Charcoal Water Filters.” Water Filters Fast, AWP Group, 17 July 2017, www.waterfiltersfast.com/5-Benefits-of-Using-Charcoal-Water-Filters_b_64.html.
Moelyaningrum, A D et al. “The Robusta Coffee Grounds Residues to Adsorb the Heavy Metal Lead (Pb) in the Water Layout Guide for Using Microsoft Word.” Journal of Physics: Conference Series, 2018, doi:10.1088/1742-6596/1114/1/012058.
Peshin, Akash. “How does Activated Charcoal Purify Water”. ScienceABC. 2 Dec 2019. www.scienceabc.com/nature/activated-charcoal-purify-water.html.
Steele, Gary. “Activated Carbon Adsorption Mechanism” 15 April 2015. Acarbons.www.acarbons.com/activated-carbon-adsorption-mechanism/
“What is activated charcoal and why is it used in filters?” 1 April 2000. HowStuffWorks.com. Science.howstuffworks.com/evnrionmental/energy/question209.htm>