Jerry Jiang and Wayne Bao. Year 2 Research.
Abstract
As water security concerns grow, more research must be conducted on energy-efficient ocean desalination techniques. One such contender is the electrocoagulation process, which has already demonstrated a high level of effectiveness in removing pollutants such as heavy metals. Our research involves exploring the effectiveness of an electrocoagulation and filter system in reducing salt concentration. We examined the efficacy of different electrode materials (zinc, iron, aluminum, copper). We discovered that an electrocoagulation + filter system can significantly decrease salt concentration in water. While the minimal salt content leftover still renders the water undrinkable, we recognize that electrocoagulation can point towards a more energy efficient desalination technique.
Introduction
Freshwater is crucial to terrestrial organisms. As the global population living in potentially severely water-scarce is expected to grow from 1.9 billion people in the mid 2010s to more than 2.7 billion people in 2050 (Boretti and Rosa, 2019), new energy-efficient alternative desalination processes need to be discovered. Furthermore, global water demand for all uses is expected to increase by 20% to 30% by 2050 while only 1% of the world’s population living in coastal areas relies on desalination, indicating the huge potential desalination will play in the future. Many current desalination plants operate through reverse osmosis, which requires a large amount of electricity and creates hyper-salinated waste water in the form of brine (Bienkowski, 2015). Thus new research must uncover alternative desalination solutions that can be cost-effective, energy-efficient, and yield higher quantities of freshwater.
The electrocoagulation filtration process is used in the water treatment process as the initial step to separate the wastewater. The filtration process is much more energy-efficient and cost-effective than conventional filtration techniques (Advantages and Disadvantages of Electrocoagulation Water Treatment, 2020). The filtration process is unique as it utilizes chemical processes like emulsion breaking, introducing oxygen and hydrogen molecules that react with the emulsified oils to form insoluble compounds, and bio-hazard reduction, oxidizing viruses and bacteria (Tetreault, 2003) instead of mechanical processes like reverse osmosis. In particular, one property of electrocoagulation that requires further investigation is the physio-chemical effect called coagulation which causes small particles in the water to destabilize and aggregate in large insoluble complexes (Bradley, 2019). The effect can be produced through introducing chemicals (Sivaramakrishnan, 2008) and when introducing electric charges to water. This effect has already been proven to be effective in removing small particles like heavy metals (El-Taweel et al. 2015), and the effect could prove useful in the gathering and removal of soluble particles like sodium and chlorine. If this is true, we believe that the electrocoagulation process could become the next mainstream desalination technique because of its energy-efficiency and cost effectiveness.
Our hypothesis is broken down into two parts. Firstly, we hypothesize that the electrocoagulation technique could prove to be an effective tool for desalination. Secondly, we hypothesize that the coagulation effect is produced because of electrocoagulation’s ability to push oxidation and reduction reactions to their natural limit. This is because a driving point of the coagulation effect is to have small particles, which are suspended in the solution due to the electrical charges, destabilize and congregate together due to the presence of opposite-charged particles. A filter driven by gravity will then be used to remove the larger insoluble complexes. Introducing electrodes of differing oxidation/reduction potentials would show the importance of oxidation and reduction reactions for the coagulation effect as the electrodes with the highest oxidation/reduction potential should result in the most amount of salt removed.
Recent research into the electrocoagulation process has generally focused on its effects to remove heavy metals, bio-waste (Uludag-Demirer and Olson 2020), and hydrocarbons (Safari et al. 2016), but there hasn’t been many studies conducted on the effectiveness of electrocoagulation for desalination. Furthemore, almost no studies have been conducted to show the effectiveness of an electrocoagulation + carbon filter process for desalination.
Materials and Methods
In this experiment, we expect the aluminum electrodes to remove the most salt as it has the highest oxidation/reduction potential out of all the electrodes we are testing at 1.66 Volts. This is followed by the zinc electrodes at 0.74 Volts, the iron electrodes at 0.45 Volts, and the copper electrodes at 0.34 Volts.
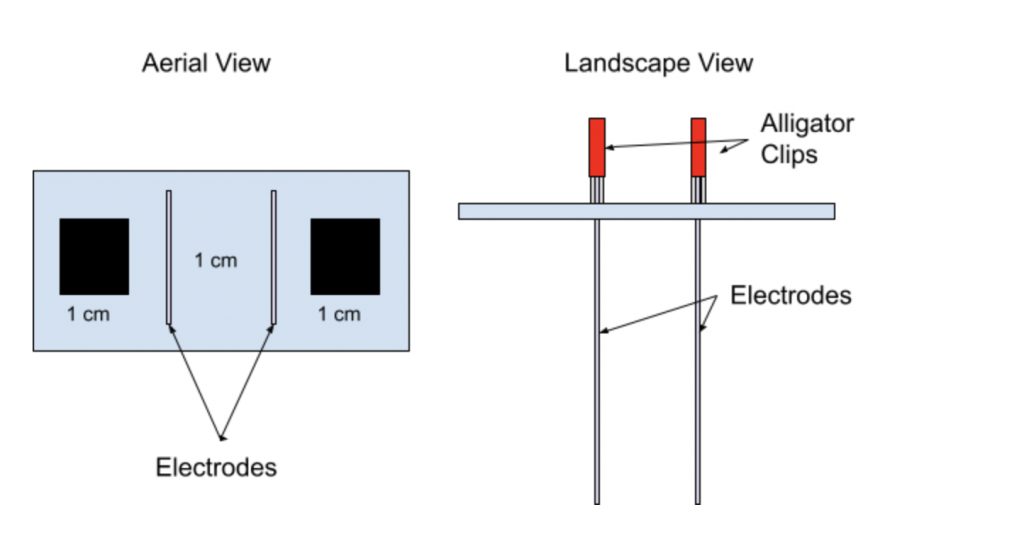
Figure 1: Model to produce the electrocoagulation process.
A balsa wood plank measuring a minimum of 6 cm by 3 cm was used in order to stabilize the electrode placement. 1 cm by 1 cm holes were added in order for hydrogen gas release. In Figure 1, a premature design of the model can be seen with a 370 ml jar being placed under the balsa wood plank. The electrodes were connected to a 9 volt alkaline battery through the use of 2 alligator clips (Goldeleway).
The main areas where reactions occur are at the cathode, anode, and in the center. At the cathode, a reduction reaction is occurring where water is gaining electrons and becomes hydrogen gas and hydroxide. The balanced equation is shown in Figure 2.
2H2O + 2e– → H2+ + 2OH–
Figure 2: Dissociation of Water.
At the anode, an oxidation reaction is occurring where the metal is losing electrons to become a metal ion. The balanced equation is shown in Figure 3.
Metal → Metal+ + e–
Figure 3: Dissociation of Metal.
At the center, a synthesis reaction occurs where the metal ion synthesizes with the hydroxide to become a metal hydroxide. The balanced equation is shown in Figure 4.
Metal+ + OH– → MetalOH
Figure 4: Synthesis of Metal Hydroxide.
The occurrence of metal hydroxides can be confirmed by looking at the final product from a copper electrode test. The solute that is produced appears to be a bluish turquoise which matches the colour of Cu(OH)2.
Ocean Water
In total, the test was run 10 times, 2 times for each electrode (iron, aluminum, copper, and zinc) (Merlan) and 2 control tests where no electrodes were used. Ocean water was collected from the Burrard Inlet area. The ocean water was filtered through two coffee filters. The MSDS of the main hazards were taken into account for this experiment and proper safety equipment was worn to ensure risks are minimized.
Refractometer Testing
The tests were separated into filtered tests and electrocoagulated tests. Electrocoagulated tests were designed to measure the effectiveness of the electrocoagulation process. The filtered tests were designed to measure the effectiveness of a carbon filter, in this case a Brita Water Filter Pitcher Advanced Replacement Filter (Brita), when added onto the electrocoagulation process. In the filtered tests, the filter was washed for 3 minutes afterwards with freshwater. The filter was also dried after each test.
To start, salinity of the original ocean water was measured using a salinity refractometer (Hallocool). The original ocean water was also run through the Brita filter. Before the tests, the electrodes were sanded with 100 grit sandpaper. The tests were run for 10 minutes, and the battery was changed after each test. After the process, the alligator clip was disconnected from the battery and the electrodes were removed from the balsa wood piece. The used electrodes were placed on a piece of paper towel, and when dry, the electrodes were sanded. The salinity of the electrocoagulated solution was measured and the salinity of the electrocoagulated solution when run through a filter was also measured.
The solution was then moved into dry plastic water bottles. The salinity of the electrocoagulation solution was measured after sitting for a day and the salinity of the electrocoagulation solution was also measured when run through a filter after sitting for a day.
Dry Weight Testing
50 ml was collected from each filtered electrocoagulated solution and kept in plastic cups. The weight of the cups were measured beforehand. The cups were left out to dry in order to collect the amount of salt that remained after each test. The weight of the salt remaining was then measured by subtracting the final weight of the cup from the original weight of the empty cup and the remaining number was multiplied by 20 to get the amount of salt in 1 litre of ocean water.
Results
The electrocoagulation results were gathered using two methods: refractometer tests, which used the unit of parts-per-thousand (ppt), and dry weight tests, which used the unit of grams/litre (g/L). The unit of g/L can be directly converted to ppt in a 1:1 ratio. Because two different tests were used to gather data, the data was displayed using two graphs.
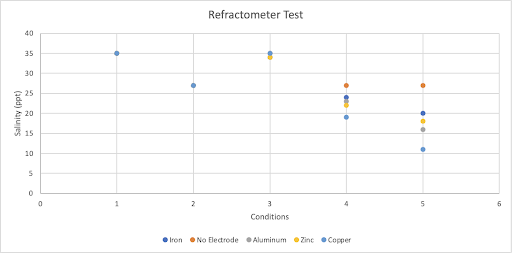
Figure 5: Electrocoagulation test results for four different electrodes and a no electrode control. Condition 1 indicates no electrocoagulation occurred and no filter was used ~ (-/-), Condition 2 indicates no electrocoagulation occurred and a filter was used ~ (-/+), Condition 3 indicates electrocoagulation occurred and no filter was used ~ (+/-), Condition 4 indicates electrocoagulation occurred and a filter was used ~ (+/+), Condition 5 indicates electrocoagulation occurred and a filter was used and the solution was measured after a day ~ (+/+) + 1 day. Results were measured using a refractometer.
In Figure 5, the values in condition 1 all had 35 ppt, the standard salinity of ocean water. In condition 2, the values all had 27 ppt. In condition 3, the iron electrodes, zinc electrodes, and aluminum electrodes all had 34 ppt. The differences start to span in the 4th condition where both electrocoagulation and a filter were applied. In the 4th test, the copper electrodes had the lowest measured value at 19 ppt followed by the zinc electrodes at 22 ppt. In the 5th condition, the differences continue to grow but the order of effectiveness stays the same. The copper electrodes had the lowest ppt at 11 followed by the aluminum electrodes at 16 ppt.
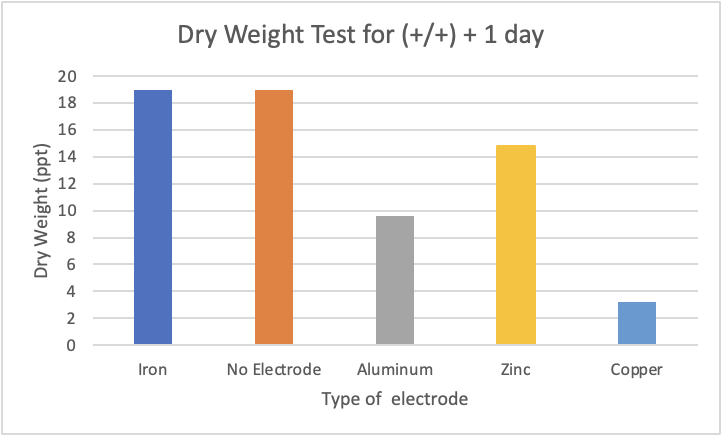
Figure 6: (+/+) + 1 day indicates electrocoagulation occurred, filter was used, and the solution was measured after a day. Results for the dry weight tests were dried and measured with a scale afterwards. The unit of grams/litre which was used to measure the weight was converted to ppt in a 1:1 ratio for more simple comparisons between tests.
In Figure 6, the copper electrode had the lowest dry weight out of all the electrodes tested 3.2 ppt compared with the second lowest, aluminum, having a value of 9.6 ppt. On the other hand, the iron test had the same dry weight as the no electrode control test, both being at 19 ppt. The results from the dry weight test are consistent with the results from the refractometer test as the order of effectiveness of electrodes remains the same.
Discussion
The goal of the experiment was to determine the effectiveness of the electrocoagulation process as an alternative desalination technique and determine the importance of oxidation/reduction reactions for the coagulation process. We expected the aluminum electrodes to remove the most salt, resulting in the lowest measurement, as aluminum has the highest oxidation/reduction potential out of all tested materials. We expected the remaining electrodes to follow in order of oxidation/reduction potential: zinc electrodes, iron electrodes, and copper electrodes.
Firstly, a comparison of particles left over from just filtering and using both the filter and electrocoagulation processes show that the electrodes were particularly effective in aiding the filtration process. For example, the filter was only capable of removing 8 ppt in the control group but after coagulation, was able to remove 16 ppt when using the copper electrode. An improvement of -3 ppt, -4 ppt, -5 ppt, -8 can be seen in using the iron, aluminum, zinc, and copper electrodes respectively in the electrocoagulation + filter test ~ (+/+) versus the no electrode test ~ (-/+). This supports our hypothesis that the electrocoagulation process can induce the coagulation effect, which changes the solubility of sodium and chlorine ions, as the filter is able to capture more particles once the electrocoagulation process occurs.
Furthermore, the efficiency of the coagulation is proven more clearly when leaving the particles in the water for 24 hours. It is observable in the copper electrode trial that it improved the efficacy from 19 ppt to 11 ppt. An improvement of -4, -7, -4, -8 can be seen in using the iron, aluminum, zinc and copper electrodes in the electrocoagulation + filter + 1 day test ~ (+/+) + 1 day respectively versus the electrocoagulation + filter test ~ (+/+). In theory, this makes sense as smaller insoluble particles that may not be captured by the filter are able to sink to the bottom of the container once more time is given so the resulting filtered solution will be clearer.
Our results indicate that, although differing oxidation/reduction reactions do influence the results, there may be more chemical property influences that affect the efficiency of salt removal. This is because the electrodes that removed the most salt from water were the copper electrodes, which also had the lowest oxidation/reduction potential. This was further backed up by the dry weight tests which resulted in the same order as the refractometer tests. One possible reason for the efficiency of copper electrodes in the electrocoagulation process is because copper has a high conductivity (Properties of Copper). Conductivity measures how much current a material can carry (Marie 2020). Copper’s high conductivity means that the copper electrodes may be able to introduce more ions into the water compared with other electrodes.
The first significant error present was we reused filters between tests. This may have increased the chance of contamination between tests. Extra salt that was not removed from the filter during the washing period may have ended up in the next test, influencing the results. In a perfect experiment, a new filter would be used for each test and more samples should have been collected to reduce the effects of outliers. Furthermore, our model may not be effective for a commercial-scale operation as the electrodes gradually lose mass as the process continues (Ashraf et al. 2019) and the potential of electrodes is slowly reduced as floc and other ions reduce the available surface hour for electrocoagulation. Full-time assistance would be required to sand and create more surface area under our model, detracting from the value of the process.
The results from the experiment were a result of months of troubleshooting and exploration. We succeeded in troubleshooting our model in order to generate the coagulation effect. In testing our hypothesis, we discovered that the electrocoagulation process when applied with a carbon filter can be used to remove salt from ocean water with our best electrodes, the copper electrodes, being able to remove 90.86% of the salt from the water. A comparison with other desalination techniques was not possible as our process removed salt from the water while other techniques like reverse osmosis removed fresh water from salt water, creating a hyper salinity waste product. We were also not able to make a conclusion on how our work compared to other electrocoagulation results as there were no other papers that focused on a similar method and objective. We also discovered that oxidation/reduction potentials do play a part in producing the coagulation effect but there are other chemical properties that may be more essential for producing the coagulation effect like conductivity. Future research could work to better understand the chemical properties that produce the coagulation effect such as conductivity, testing the effectiveness of high conductivity metals like gold and copper vs lower conductivity metals like tin. Research could also explore the effectiveness of alloys of different metals for removing salt from ocean water as different ions introduced together may remove different kinds of particles. Although the electrocoagulation process has been widely tested for its effects to remove heavy metals, bio-waste, and hydrocarbons, our research shows the possibility for additional use in the desalination process.
References
Advantages and Disadvantages of Electrocoagulation Water Treatment. 2 Apr. 2020, https://chemtech-us.com/advantages-and-disadvantages-of-electrocoagulation-water-treatment/.
Al-Raad, A., et al. “Treatment of Saline Water Using Electrocoagulation with Combined Electrical Connection of Electrodes.” Processes, vol. 7, no. 5, Apr. 2019, p. 242. DOI.org (Crossref), doi:10.3390/pr7050242.
Ashraf, Syeda Nishat, et al. “Electrocoagulation for the Purification of Highly Concentrated Brine Produced from Reverse Osmosis Desalination of Coal Seam Gas Associated Water.” Journal of Water Process Engineering, vol. 28, Apr. 2019, pp. 300–10. DOI.org (Crossref), doi:10.1016/j.jwpe.2019.02.003.
Bienkowski, B. “Desalination Is an Expensive Energy Hog, but Improvements Are on the Way.” The World from PRX, 15 May 2015, https://www.pri.org/stories/2015-05-15/desalination-expensive-energy-hog-improvements-are-way.
Boretti, A., and L. Rosa. “Reassessing the Projections of the World Water Development Report.” Npj Clean Water, vol. 2, no. 1, Dec. 2019, p. 15. DOI.org (Crossref), doi:10.1038/s41545-019-0039-9.
Bradley, Eric. Wastewater Coagulation. 19 Dec. 2019, https://www.dober.com/water-treatment/resources/wastewater-coagulation.
Electrocoagulation vs. Chemical Coagulation, Powell Water Systems, Inc. https://powellwater.com/electrocoagulation-vs-chemical-coagulation/. Accessed 9 Apr. 2021.
El-Taweel, Yehia A., et al. “Removal of Cr(VI) Ions from Waste Water by Electrocoagulation Using Iron Electrode.” Egyptian Journal of Petroleum, vol. 24, no. 2, June 2015, pp. 183–92. DOI.org (Crossref), doi:10.1016/j.ejpe.2015.05.011.
Emulsions: When Oil and Water Do Mix. https://www.ift.org/news-and-publications/food-technology-magazine/issues/2013/august/columns/processing-1. Accessed 9 Apr. 2021.
Malakootian, M., and N. Yousefi. “THE EFFICIENCY OF ELECTROCOAGULATION PROCESS USING ALUMINUM ELECTRODES IN REMOVAL OF HARDNESS FROM WATER.” Iran. J. Environ. Health. Sci. Eng., vol. 6, no. 2, 2009, pp. 131–36.
Marie, A. “Understand Electrical Conductivity.” ThoughtCo, 29 Jan. 2020, https://www.thoughtco.com/definition-of-electrical-conductivity-605064.
Milne, Nicholas A., et al. “Chemistry of Silica Scale Mitigation for RO Desalination with Particular Reference to Remote Operations.” Water Research, vol. 65, Nov. 2014, pp. 107–33. DOI.org (Crossref), doi:10.1016/j.watres.2014.07.010.
“Chemistry of Silica Scale Mitigation for RO Desalination with Particular Reference to Remote Operations.” Water Research, vol. 65, Nov. 2014, pp. 107–33. DOI.org (Crossref), doi:10.1016/j.watres.2014.07.010.
Properties of Copper. https://copperalliance.org.uk/about-copper/copper-alloys/properties-copper/. Accessed 17 Apr. 2021.
Safari, S., et al. “Electrocoagulation for COD and Diesel Removal from Oily Wastewater.” International Journal of Environmental Science and Technology, vol. 13, no. 1, Jan. 2016, pp. 231–42. DOI.org (Crossref), doi:10.1007/s13762-015-0863-5.
Sivaramakrishnan, C. “Effluent Treatments, Chemical Coagulation, Water Treatment.” Fibre2fashion.Com, Feb. 2008, http://www.fibre2fashion.com/industry-article/3095/effluent-treatments-coagulation.
Tetreault , A. Electrocoagulation Process for Wastewater Treatment. 081678364, Meat & Livestock Australia Limited, Feb. 2003, p. 30, https://www.ampc.com.au/uploads/cgblog/id172/ENV_2003_Electrocoagulation_process_for_wastewater_treatment.pdf.
Uludag-Demirer, S., and N. Olson. “Techno-Economic Analysis of Electrocoagulation on Water Reclamation and Bacterial/Viral Indicator Reductions of a High-Strength Organic Wastewater—Anaerobic Digestion Effluent.” Sustainability, Mar. 2020.
Water Facts – Worldwide Water Supply . Bureau of Reclamation, 4 Nov. 2020, https://www.usbr.gov/mp/arwec/water-facts-ww-water-sup.html#:~:text=97%25%20of%20the%20earth’s%20water,most%20industrial%20uses%20except%20cooling).&text=3%25%20of%20the%20earth’s%20water%20is%20fresh.
Zaied, B. K., et al. “A Comprehensive Review on Contaminants Removal from Pharmaceutical Wastewater by Electrocoagulation Process.” Science of The Total Environment, vol. 726, July 2020, p. 138095. DOI.org (Crossref), doi:10.1016/j.scitotenv.2020.138095.