Nicolas Deporcq-Pomerleau, Year 2 Research.
Abstract
As sales of products labelled as “antibacterial” rise, discussions about the necessity of said products are also brought up. With the 2016 ban of Triclosan, numerous studies have been conducted to test these products. In this experiment, different household antibacterial products were used on E. Coli samples to see if their use would effectively prohibit bacterial growth. The products tested were antibacterial hand soap, disinfecting wipes, antibacterial dish soap, and hand sanitizer gel. Concentrations of 100%, 50%, and 25% of these substances were also tested. The experiment concluded that most products can effectively prohibit E. Coli when used, but that this effectiveness goes down when the product concentration is lowered. Benzalkonium chloride was shown to be the most effective active ingredient, but further research is needed to confirm which active ingredients are most effective.
Introduction:
With the rapid global expansion of the COVID-19 pandemic starting in late 2019, people around the world have rushed to get their hands on household products labelled as “antibacterial”. According to the British data management company Kantar, “Sales of hand sanitiser increased by 255% in February [2020]”. This trend is not new, as household hygiene products with antibacterial compounds have been becoming increasingly popular over the years.
However, discussions about the necessity of antibacterial hygienic products have been recurring, especially after the ban of Triclosan in 2016, an antibacterial compound which was once widely-used in many products. In this same report from the FDA, it is mentioned that “Consumers may think antibacterial washes are more effective at preventing the spread of germs, but we have no scientific evidence that they are any better than plain soap and water”. Furthermore, this same report mentions suspicions that antibacterial soap containing compounds such as Triclosan may lead to hormonal issues or bacterial resistance. After hearing these things, one may start questioning the necessity of antibacterial compounds in hygiene products.
In this study, different household antibacterial products were used on an E. Coli K12 culture plate in an effort to test effectiveness at prohibiting bacterial growth. A variety of products containing different non-biological active antibacterial ingredients such as Lactic acid and Ethyl alcohol will be tested.
Materials and Methods:
To prepare the experiment, petri dishes with agar were first made. Nutrient agar (3g beef, 5g gelatin, 15g agar) (Carolina). After being pressure sterilized at 121℃ and 15 PSI, it was microwaved at 70% power in one minute intervals. After being melted, this was poured into 25 petri dishes. After the gels hardened, E. Coli K12 (Merlan Scientific) was swabbed uniformly onto one of the dishes. This dish was then placed in an incubator for a week. Throughout the experiment, bacteria growth taken from this one plate was used.
For each antibacterial product in question, 4 petri dishes were used. Each plate was first subject to an E. Coli suspension before coming in contact with the product. To do this, E. Coli culture was swabbed off of the plate mentioned earlier, and placed into a microtube with 1.5ml of sterilized water. The microtube was mixed thoroughly, and continued to be diluted until it’s turbidity matched the turbidity of a McFarland 0.5 standard (Gibson Bioscience). This resulted in a bacteria suspension per microlitre. 100μl of this diluted substance was placed onto each of the 24 plates and left to dry for 20 minutes.
A disk diffusion method was used to measure antibacterial resistance. On each plate, 4 sterile Halo assay disks were placed in equal distance. (One control with only water, one with 100% concentration of the product in question, one with 50% concentration, and one with 25% concentration.) The products were diluted with sterile water. The products in question were Antibacterial Hand Soap (Dial) with Benzalkonium chloride as the active ingredient, Disinfecting Wipes (Lysol) with Benzalkonium chloride as the active ingredient, Dish Soap (Palmolive) with Lactic acid as the active ingredient, and Hand Sanitizer Gel (One Step) with Ethyl alcohol as the active ingredient. 20μl of each substance was pipetted on each paper disk, then placed onto the petri dishes. After all disks were plated, the petri dishes were left in an incubator for a week before being measured.
The effectiveness was measured by checking the size of the zone of inhibition, which is the zone where bacteria growth was inhibited on the disks. The diameter of each zone was measured three times with a ruler, then averaged out. Measures were taken in millimeters.
Results:
The controlled disk with water had no noticeable effect on the bacteria surrounding it, creating no zone of inhibition. Higher concentration of product was shown to create a larger zone of inhibition. In every sample, as the concentration lowered from 100% to 50% and 50% to 25%, the size of the zone of inhibition also shrank significantly. The hand soap created the largest zone of inhibition, followed by the antibacterial wipes, the dish soap, and the hand sanitizer. However, it is to be noted that every disk and concentration of hand sanitizer gel produced no zone of inhibition.
The results for each product are as follows. The hand soap produced a zone of inhibition with the diameter of 24.62mm with 100% concentration, 22.23mm with 50% concentration, and 19.34mm with 25% concentration. This shows that a 100% to 50% concentration drop caused the zone of inhibition to decrease by 10%, and that a 50% to 25% concentration drop caused the zone of inhibition to decrease by 13 %. The disinfecting wipes produced a zone of inhibition with the diameter of 23.78mm with 100% concentration, 21.84mm with 50% concentration, and 20,12mm with 25% concentration. This shows that a 100% to 50% concentration drop caused the zone of inhibition to decrease by 8% and that a 50% to 25% concentration drop caused the zone of inhibition to decrease by 7%. The dish soap produced a zone of inhibition with the diameter of 22.45mm with 100% concentration, 19.61mm with 50% concentration, and 16.12mm with 25% concentration. This shows that a 100% to 50% concentration drop caused the zone of inhibition to decrease by 13% and that a 50% to 25% concentration drop caused the zone of inhibition to decrease by 18%. The hand sanitizer gel produced no zone of inhibition with every concentration level. All control disks (0% product and only water) produced no zone of inhibition.
These numbers show that when diluted with water to 50%, the effectiveness of products went down an average of 10.34% compared to a 100% concentration. When diluted to 25% concentration, the effectiveness of products went down an average of 12.67% compared to a 100% concentration. (This calculation was conducted disregarding the values produced by the hand sanitizer, as it is an outlier.)
Figures & Tables:
Tables showing the diameter of the zone of inhibition for the various products and concentrations tested. Values are all shown in millimeters. (The average of the three measurements taken of each zone of inhibition is put in columns from 1-6.)
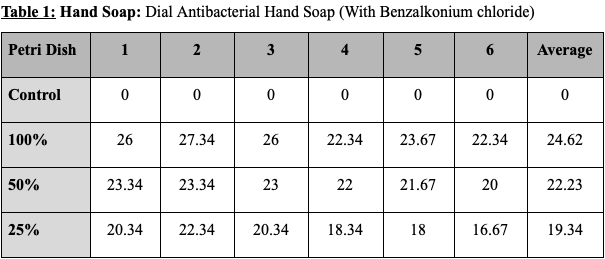
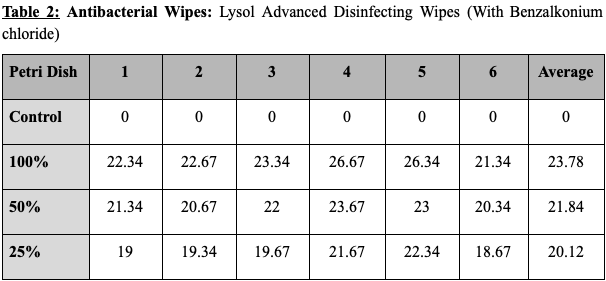
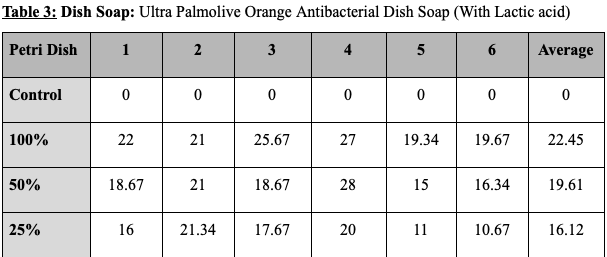
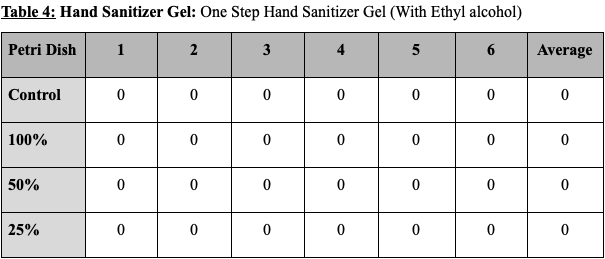
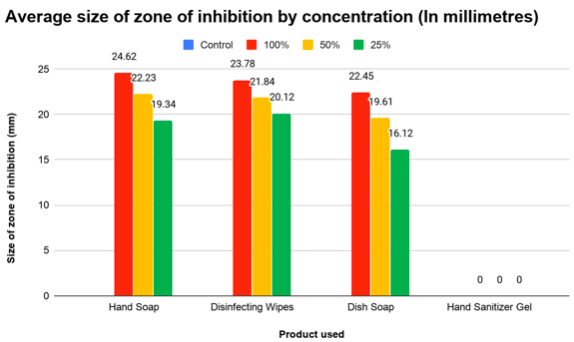
Figure 1: Graph comparing the average size of zone of inhibition by product and concentration tested.
Discussion:
The results have shown that higher concentrations of an antibacterial product will lead to a larger zone of inhibition, which means that less E. Coli was able to grow. Not all antibacterial products have the same level of effectiveness, as some allow more antibacterial growth. The hand soap was found to be most effective at preventing bacterial growth, producing the biggest zone of inhibition. This is followed by the antibacterial wipes, then the dish soap.
Dilution was continuously shown to reduce effectiveness, with zones of inhibition shrinking over 10% with each level of dilution. Within the products tested, the dish soap was most prone to this, losing 13% of its effectiveness when concentration was reduced from 100% to 50%, then an additional 18% once concentration was reduced from 50% to 25%. This suggests that keeping products in full concentration is necessary to effectively prohibit bacterial growth. Concentration of these products is crucial as reduced effectiveness can cause serious complications in a situation such as when sterilizing medical equipment.
The results of this study suggest that antibacterial products are effective to a certain degree at prohibiting E. Coli growth, but that this effectiveness is cut down when said products are diluted with water. Different active ingredients are shown to result in various degrees of effectiveness. As the product which showed most effectiveness was hand soap that had Benzalkonium chloride as an antibacterial agent, this study may suggest that Benzalkonium chloride is more effective compared to Lactic acid and Ethyl alcohol in prohibiting E. Coli growth. Furthermore, since gel hand sanitizer with Ethyl alcohol as an active ingredient did not prohibit any bacterial growth, this study may suggest that Ethyl alcohol is not effective at prohibiting E. Coli K12 bacterial growth. The possibility of a systematic error with the gel hand sanitizer samples is unlikely, as all 18 disks deposited on six different petri dishes produced no results. Previous experiments such as one by the Centres for Disease Control and Prevention have proved the efficiency of Ethyl alcohol in eliminating E. Coli bacteria when used in concentrations of 40% to 100%. (CDC, 2008) This could point to the hypothesis that the concentration used in the hand gel tested in this experiment was simply too low to effectively inhibit E. Coli K12 growth.
Multiple larger-scale studies have been conducted around the world in order to determine if soap with antibacterial ingredients are truly effective at reducing bacterial contamination, and if their results are significant in comparison to traditional soap. Many come to a similar conclusion, that antibacterial soaps are “no more effective than plain soap at preventing infectious illness symptoms and reducing bacterial levels on the hands”, as it is shown in a study conducted for the Infectious Disease Society of America. (Allison et al, 2007) This considered, another future research that could be conducted to further this one would be to test traditional soaps, and compare their results to those produced by antibacterial soaps.
Since this research was done on a small scale, with four products using three different active antibacterial ingredients, and repeated six times, a bigger range of products and more repeats would be needed before making concrete conclusions. A future research that could be pursued following this research would be testing various different products with the same active ingredients to see if some ingredients are more effective than others. After doing so, it could be seen if the suggestion from this research that Ethyl alcohol is ineffective and that Benzalkonium chloride is very effective is a recurring pattern or not.
After conducting this research, it can be suggested that not all products labeled as “antibacterial” are as efficient as they are often thought to be. Especially with one of the samples studied inhibiting no bacterial growth in any of its samples, it can be thought that some products are just not worth purchasing and using. This highlights the importance of properly researching the ingredients and efficiency of a product before putting it to use, especially during times of vulnerability.
References:
Allison E. Aiello, Elaine L. Larson, Stuart B, Levy. “Consumer Antibacterial Soaps: Effective or Just Risky?.” Clinical Infectious Diseases. Volume 45. Supplement_2. (2007): S137–S147. https://doi.org/10.1086/519255
Office of the Commissioner. FDA.gov “FDA Issues Final Rule on Safety and Effectiveness of Antibacterial Soaps.” U.S. Food and Drug Administration (FDA), 11/02/2016. Online article. Accessed 04/30/2020. https://www.fda.gov/news-events/press-announcements/fda-issues-final-rule-safety-and-effectiveness-antibacterial-soaps
McKevitt, F. Kantar.com. “Fastest Growth since November Puts UK Grocery Market in Better Health.” Kantar Group and Affiliates, 03/03/2020. Online article. Accessed 04/30/2020. www.kantar.com/Inspiration/FMCG/Fastest-growth-since-November-puts-UK-grocery-market-in-better-health.
Centers for Disease Control and Protection. (2008). Disinfection and Sterilization: Chemical Disinfectants. CDC.gov.