Sean Hong, Year 2 Research.
Abstract
Biofilms are a common formation for bacteria and algae to grow in and they appear in a multitude of places, from oceans to intestinal tracts. This study aimed to test the effects that microplastics may have on biofilms. This was done by growing samples of Lactococcus lactis in a YPD agar broth in plastic wells, while also adding a concentration of microplastics between the different trials. Of the 5 trials conducted, other than the contamination check and the control, there seemed to be no significant differences in visible growth. However, comparing the growth of the microplastic trials and a test run of an assay prior, it is possible that microplastics may stimulate growth in fluid environments.
Introduction
Biofilms are a very common formation for microorganisms to form. They are present in natural environments like in oceanic biomes and forests, as well as artificial facilities, such as farms and water treatment plants. Due to it’s common nature and widespread formation they can become very difficult to manage, creating a wide variety of problems like, being the source of infectious bacteria, or poisoning widely used water supplies (Flemming, and Hans-Curt. 1993). Microplastics are generally classified as any plastic fragment that has a maximum length smaller than 5mm, however, there are still a variety of different materials and sizes that they can be found in. These microplastics can be from cosmetic or industrial sources, but most microplastics found in nature are fragments from waste products (Galafassi, Silvia, et al. 2019). People also come into contact with microplastics either from the environment in products like seafood or in plastics used in the food industry like nylon tea infusers (Smith, Madeleine, et al. 2018, Hernandez, Laura M., et al. 2019). The World Health Organization has deemed that the current concentrations of microplastics do not pose any immediate threats to human health (World Health Organization; 2019). Yet, in the past decade, the concentration of microplastics in the environment has increased tremendously (Karlsson, Therese M., et al. 2018). These kinds of particles can bioaccumulate and will spread through ecosystems around the world, for example, in the upper layers of oceanic biomes (Saley, A.m., et al. 2019). Being that the right biofilms have the capability to do lots of harm, it is necessary to gather information on the interactions that microplastics have with them so we can better understand how it will affect the future. The goal of this paper is to infer how microplastics may affect bacterial colonies, by assessing how biofilm colonies of Lactococcus lactis grow in response to varying degrees of microplastic concentrations.
Materials and Methods
Microplastic samples were produced using nylon tea bags (Yosoo 100pcs Nylon Teabags). Each tea bag was separated along parallel seams (See figure 1.) by hand and then cut into fourths. Processing the teabags into strips increases the length of the seams in which microplastics can be released. The nylon strips were then processed in a blender with about 50 mL of water, submerging the blades and nylon strips. The nylon strips were then blended with intermittent pulses for 3 minutes. The blended solution was decanted into a mason jar with an intact nylon tea bag placed halfway over the lid as a filter. The solution passed through the nylon filter 3 times to strain out larger macroplastic threads (~5mm), and the resultant solution was then decanted into a 100mL beaker and placed on a heating element (Bipee Magnetic Stirrer SH-2) at max heat for 25 minutes. This achieved three things: breaking up smaller microplastic fragments, sterilizing the microplastic samples, and reducing the volume of water in the microplastic solution. After the time elapsed, the solution was left for 10 minutes to cool down. The concentrated solution was then transferred using an S-1000 micropipette into 1.5mL centrifuge tubes, each holding 1.0mL of the solution. To compare the turbidity of both the microplastic sample and the clear sample, another 1.5mL centrifuge tube with 500µL of distilled water was used.
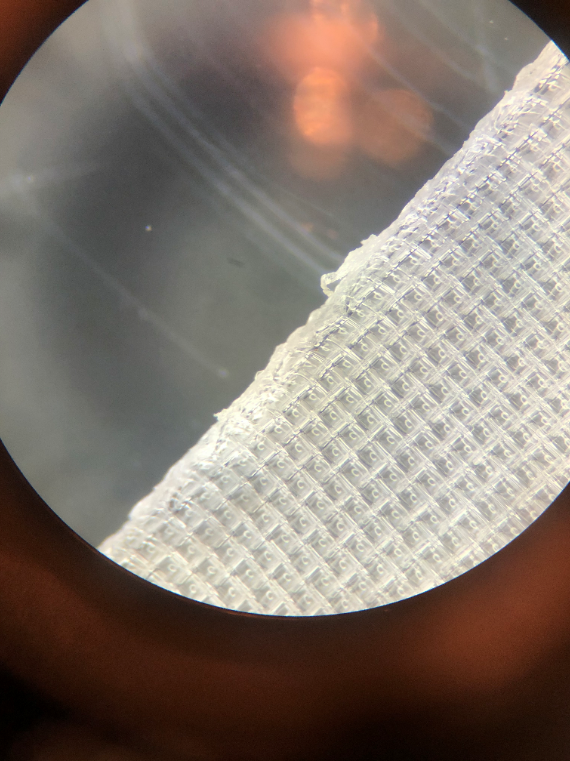
Figure 1: Nylon tea bag mesh taken at 50x magnification.
Samples of the bacterial strain, Lactococcus lactis, from Carolina Biological were used to create the biofilm in a liquid assay. Pre-sterilized plastic wells, with each plate holding 24 wells in a 4×6 arrangement were used to incubate and conduct the experiment. The bacteria were grown in a YPD agar broth in an incubator at a temperature setting of 2. Four trials were conducted on the plate with triple redundancy. Wells of 2mL YPD agar broth was left to indicate any traces of contamination. All L. lactis assays were grown in the YPD agar broth with the microplastics and bacteria added in. The control used no microplastics with 400µL of L. lactis diluted using the McFarland Standard. Trial 1 used 400µL at 10% of the total microplastic concentration along with 400µL of L. lactis using the McFarland Standard. Trial 2 used 400µL at 50% of the total microplastic concentration along with 400µL of L. lactis using the McFarland Standard. Trial 3 used 400µL at 100% of the total microplastic concentration along with 400µL of L. lactis using the McFarland Standard. A trial of only microplastics was originally planned but was decided against after initial observations showed negligible turbidity when the microplastics were added to the agar broth. The wells were then placed on top of a gyrating platform and covered to incubate for one day.
Results
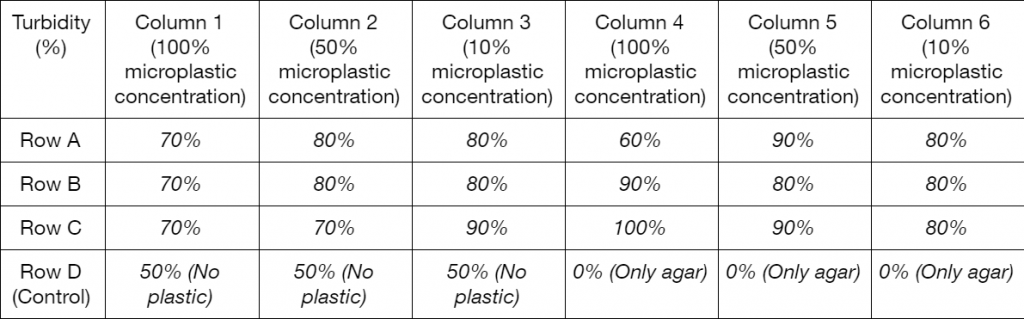
Due to complications during the drafting phase of the experiment, further work on the project was much slower than expected. From the data that was collected, it is not possible to draw a definitive conclusion. To start with the procedure of creating nylon microplastics. By using a microscope it was possible to view possible areas where particles could leach, (see Figure 1) but only boiling the particles yielded nothing visible. After employing the blender to process the nylon, there were many pieces that were identified as microplastics. Comparing the growth of the experiment with a trial to determine the effectiveness of YPD agar with L. lactis shows a significant reduction of growth. Of the 24 wells in the plate, with triple redundancy for each trial, there were 5 trials being conducted (Figures 2-4). The empty YPD agar broth wells showed no signs of growth, indicating a lack of contamination. Yet the rest of the plates, each with varying amounts of microplastics had much more uniform turbidity and growths when compared to the previous test trials. Even with an increase of microplastics the general formation of each biofilm was very comparable with the last (Table 1.).

Figure 2: 24 wells of YPD agar broth with grown L. lactis samples. Wells D4-D6 are used to check for contamination. Wells D1 through D3 are the control with no microplastics. Wells A3, B3, C3, A6, B6, C6 contain 10% microplastic concentration. Wells A2, B2, C2, A5, B5, C5 contain 50% microplastic concentration. Wells A1, B1, C1, A4, B4, C4 contain 100% microplastic concentration.

Figure 3: Left-most column: wells A4, B4, C4 (100% microplastics). Middle column: wells A5, B5, C5 (50% microplastics). Right-most column: wells A4, B4, C4 (10% microplastics)

Figure 4: Left-most column: wells A1, B1, C1 (100% microplastics). Middle column: wells A2, B2, C2 (50% microplastics). Right-most column: wells A3, B3, C3 (10% microplastics)
Table 1. Qualitative observational data of the turbidity of each well as a percentage 0% being the turbidity of a clear well (D4 through 6), 50% being set as the 0% microplastic control (D1 through D3), and 100% of the most turbid well (set as C4).
Discussion
The experiment is inconclusive with the current observations, as the resources to accurately measure the differences between each trial were inaccessible. The original hypothesis, that microplastics will adversely affect the growth of biofilms of the bacteria L. lactis cannot be determined with the current data, however, there are some insights that may be abstracted from observations done by other papers. In the case of this paper each well in the microplastics trial displayed very even growths, being nearly identical regardless of microplastic quantity. This may indicate that the presence of microplastics may have distributed or improved the bacterial growth in a consistent manner. Multiple studies have observed that microplastic debris have served as surfaces for algae and microorganisms to grow on (Michels, Jan, et al. 2018, Urbanek, Aneta K., et al. 2018). One study focusing on bacteria and microplastics in cold-marine environments show that some bacteria can grow around these microplastic particles and eventually break them down (Urbanek, Aneta K., et al. 2018). This kind of interaction may explain the increase in turbidity from the trials done with microplastics versus the earlier trials done without them (Figure 2, Figure 5).

Figure 5: Liquid assay of L. lactis done in YPD agar broth. Left-most column: wells A6, B6, C6, D6 filled with 400µL of L. lactis bacteria at McFarland Standard. Middle column: wells A5, B5, C5, D5 filled with 200µL of L. lactis bacteria at McFarland Standard. Right-most column: wells A3, B3, C3 serve as control (No bacteria)
A new hypothesis based on the observations from this experiment is that rather than disrupting the formation of a film, the microplastics act as growing surfaces which assist in the binding and distribution of the bacteria. To deduce whether or not the turbidity is truly caused by the bacteria growing on free floating fragments of microplastics, extracting the turbid solution and growing it in a separate assay can show whether or not the bacteria is within the turbid medium. Other tests may involve changing the type of plastics or microorganisms used in this experiment. Another vein of this research can focus on how microplastics carry around bacteria into other environments, by acting as dispersible particulates that carry aggregates of bacteria. If microplastics do allow for microorganisms to grow around them it would be recommended to keep a close look at how microplastics are distributed within the environment. There may be a trend of different species of microorganisms moving to new ecosystems in much larger quantities, or of contaminants from industrial zones making their way to urban populations with increasing amounts.
References:
Flemming, and Hans-Curt. “Biofilms and Environmental Protection.” Water Science and Technology, IWA Publishing, 1 Apr. 1993, iwaponline.com/wst/article/27/7-8/1/24516/Biofilms-and-Environmental-Protection.
Galafassi, Silvia, et al. “Plastic Sources: A Survey across Scientific and Grey Literature for Their Inventory and Relative Contribution to Microplastics Pollution in Natural Environments, with an Emphasis on Surface Water.” Science of The Total Environment, vol. 693, 25 Nov. 2019, p. 133499., doi:10.1016/j.scitotenv.2019.07.305.
Hernandez, Laura M., et al. “Plastic Teabags Release Billions of Microparticles and Nanoparticles into Tea.” Environmental Science & Technology, vol. 53, no. 21, 2019, pp. 12300–12310., doi:10.1021/acs.est.9b02540.
Karlsson, Therese M., et al. “The Unaccountability Case of Plastic Pellet Pollution.” Marine Pollution Bulletin, vol. 129, no. 1, Apr. 2018, pp. 52–60., doi:10.1016/j.marpolbul.2018.01.041.
Michels, Jan, et al. “Rapid Aggregation of Biofilm-Covered Microplastics with Marine Biogenic Particles.” Proceedings of the Royal Society B: Biological Sciences, vol. 285, no. 1885, 2018, p. 20181203., doi:10.1098/rspb.2018.1203.
Saley, A.m., et al. “Microplastic Accumulation and Biomagnification in a Coastal Marine Reserve Situated in a Sparsely Populated Area.” Marine Pollution Bulletin, vol. 146, Sept. 2019, pp. 54–59., doi:10.1016/j.marpolbul.2019.05.065.
Smith, Madeleine, et al. “Microplastics in Seafood and the Implications for Human Health.” Current Environmental Health Reports, vol. 5, no. 3, 2018, pp. 375–386., doi:10.1007/s40572-018-0206-z.
Urbanek, Aneta K., et al. “Degradation of Plastics and Plastic-Degrading Bacteria in Cold Marine Habitats.” Applied Microbiology and Biotechnology, vol. 102, no. 18, Nov. 2018, pp. 7669–7678., doi:10.1007/s00253-018-9195-y.
World Health Organization “Microplastics in drinking-water.” Geneva: World Health Organization; 2019. Licence: CC BY-NC-SA 3.0 IGO